Preclinical data on lead coronaviruses antibody
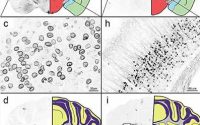
Adagio Therapeutics today published in vitro and in vivo data in Science on its lead antibody candidate, ADG2, which demonstrated similar or higher potency against SARS-CoV-2 compared to other monoclonal antibodies (mAbs) in clinical development and strong binding to all known SARS-CoV-2 variants. Uniquely, ADG2 also showed broad and potent neutralization against a range of sarbecoviruses that pose a threat to humans and protective efficacy in murine models of SARS and COVID-19. The data show that ADG2 effectively binds to and has the potential to protect against common circulating SARS-CoV-2 variants as well as future SARS-related viruses with pandemic potential. Adagio’s half-life engineered version of ADG2, called ADG20, could offer protection against COVID-19 for up to a year. Adagio expects ADG20 to enter Phase 1 clinical studies in early 2021.
“These studies demonstrate our lead antibody shows comparable or higher neutralization potency against SARS-CoV-2 than leading antibodies currently in development for COVID-19 and binds effectively to all of the most commonly circulating SARS-CoV-2 variants, indicating that it should not be affected by known resistance mutations. Unlike most antibodies currently in clinical development or approved for emergency use, ADG20 binds to a highly conserved epitope, so we believe it will also be effective against future emerging SARS-CoV-2 strains and related pre-emergent sarbecoviruses,” said Laura Walker, Ph.D., chief scientific officer of Adagio.
Potency and breadth of coverage
- ADG2 (the precursor to ADG20) provided complete protection against severe SARS-CoV-2 and SARS-CoV disease in murine models of COVID-19 and SARS, respectively.
- When compared to other mAbs in development or approved for emergency use, ADG2 showed similar or higher potency against SARS-CoV-2 in two authentic neutralization assays.
- ADG20 demonstrated strong binding to all commonly circulating SARS-CoV-2 variants.
- ADG2 also showed broad and potent neutralizing activity against SARS-CoV and two SARS-related coronaviruses currently known to be circulating in bat populations (WIV-1 and SHC014).
- The epitope targeted by ADG2 is highly conserved across clade 1 sarbecoviruses, and ADG2 binds with high affinity to a large panel of clade I sarbecovirus receptor binding domains (RBDs).
Viral resistance variants
- ADG2 binds with high affinity to more than 30 of the most frequently observed SARS-CoV-2 RBD variants reported in the GISAID database, including the known variants resistant to other monoclonal antibodies in development or approved for emergency use.
- Notably, no mutations have been reported at key ADG20 contact residues in full length viral genomic sequences (>152,000) of SARS-CoV-2 included in the GISAID database as of December 9, 2020, suggesting a low risk of pre-existing resistance to ADG20 in the clinic.
Other enhanced attributes
- In vitro engineering of ADG2 avoided many of the common pitfalls associated with monoclonal antibody enhancements. Specifically, ADG2 demonstrated favorable biophysical properties in a series of in vitro assays that have been shown to be predictive of downstream behaviors such as serum half-life, ease of manufacturing, ability to formulate to high concentrations, and long-term stability.
- Although not included in the pre-publication, Adagio also engineered ADG2 to extend serum half-life and enhance mucosal localization.
Source: Read Full Article