Understanding interactions between drugs and viruses is key to readiness for variants, next pandemic
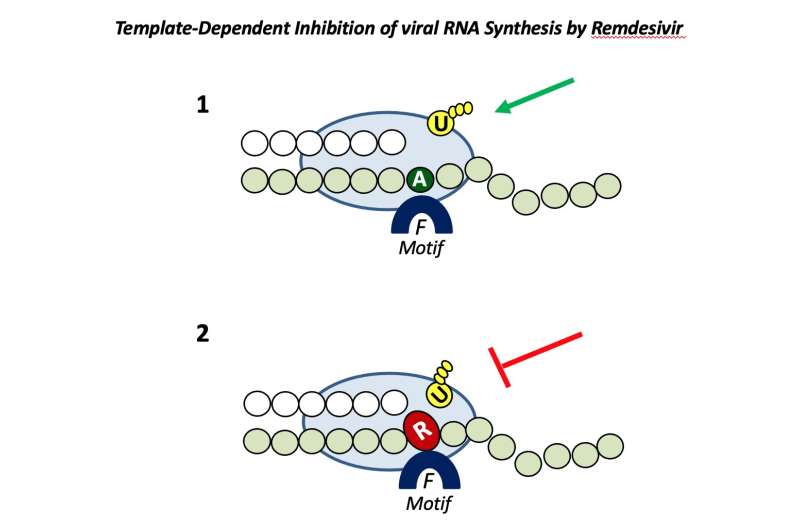
New research shows for the first time why the antiviral drug remdesivir works against some viruses but not others—a finding that improves our understanding of how antiviral drugs interact with viruses at a molecular level, which will be key to developing the broad-spectrum therapies needed to battle against the SARS-CoV-2 pandemic and get ready to fight the next one.
The paper, published in the Journal of Biological Chemistry, reveals the inner workings of the drug remdesivir against different families of viruses. Remdesivir has been given to more than nine million COVID-19 patients worldwide, and is so far the only small-molecule antiviral drug that has received Health Canada approval to treat the disease.
Until now it was not understood why remdesivir works in lab tests against some viruses including coronaviruses, Ebola, hepatitis C and Nipah virus, but not against others such as influenza and Crimean-Congo hemorrhagic fever virus.
Matthias Götte, professor and chair of medical microbiology and immunology at the University of Alberta, noted that it all comes down to how well the drug tricks the polymerase, which is the replication engine of the virus and the target of remdesivir.
“Remdesivir is very well incorporated by the polymerase of SARS-CoV-2 and not so well by other viruses where it does not work,” he said, adding that once it is incorporated, the drug inhibits all viral polymerases tested in the study.
Now that the interactions between remdesivir and several other viruses are better understood, the next step will be to modify the compound to be better accepted by the polymerase of a broader range of other viruses.
“What we would like to have when the next pandemic strikes—and it’s not a question of if, it’s a question of when—what we need to have on the first day is a broad-spectrum antiviral agent,” Götte said.
Timing is everything
Another key to successful antivirals is timing. Clinical trials have shown that remdesivir reduces the risk of hospitalization by 87 percent when it is given early in the course of illness, but because it is currently only available intravenously, it’s sometimes given too late to have its full effect, Götte explained. The United States and Europe recently recommended remdesivir for early use in outpatients with mild to moderate disease, and Götte hopes Canada will follow suit. While vaccination remains the most important available tool against SARS-CoV-2, he said remdesivir continues to be effective against the Omicron variant and could play an important role in reducing the burden on our health system.
Götte said the new Pfizer oral antiviral drug that is awaiting Health Canada approval appears to be just as effective as remdesivir at preventing hospitalizations when given early. Another orally available antiviral drug, molnupiravir, has just been given FDA approval for use against COVID-19 in the United States, even though it appears to prevent only about 30 percent of hospitalizations. It also shows activity against other viruses, including influenza.
For Götte, the more antiviral options we have available, the better. If a new virus strikes, it will take time to develop a vaccine, and treatments will be needed around the world while we wait. The ideal situation would be to treat patients with more than one antiviral at a time, much like the “cocktails” used for HIV and hepatitis C.
Source: Read Full Article


