Alterations in mitochondrial dynamics may cause inflammation and muscular atrophy
Mitochondria are essential cellular components that act as powerhouses. The fusion of two or more mitochondria, and the fission of a mitochondrion into two units are common phenomena known as “mitochondrial dynamics” and both processes are necessary for the proper functioning of these structures, as well as of the cell itself.
Researchers at IRB Barcelona, led by Dr. Antonio Zorzano, professor at the faculty of biology of the University of Barcelona (UB) and a member of CIBERDEM, have described a new molecular mechanism related to alterations in mitochondrial dynamics and inflammation, which results In muscular atrophy. Researchers have found that blocking either mitochondria fusion or fission triggers an inflammatory process, although this reaction arises due to different mechanisms.
“Chronic inflammation is one of the processes that affect our health, given that it is linked to a wide range of diseases, such as diabetes, Alzheimer’s, Parkinson’s, and cancer, not to mention aging. This is why it is important to understand it, so that we can tackle it and prevent related disorders,” explains Dr. Zorzano, the head of the Complex Metabolic Diseases and Mitochondria Laboratory at IRB Barcelona.
The phenomenon, as described in the journal Nature Communications, has been observed in muscle cells in culture, as well as in mouse muscles used in experimental models. The type of inflammation described is known as “sterile inflammation,” as it is not linked to an infectious process. These studies open the way to exploring the role of alterations in mitochondrial dynamics with respect to the development of human diseases, especially those diseases that affect muscles.
Mechanisms that stimulate inflammatory processes
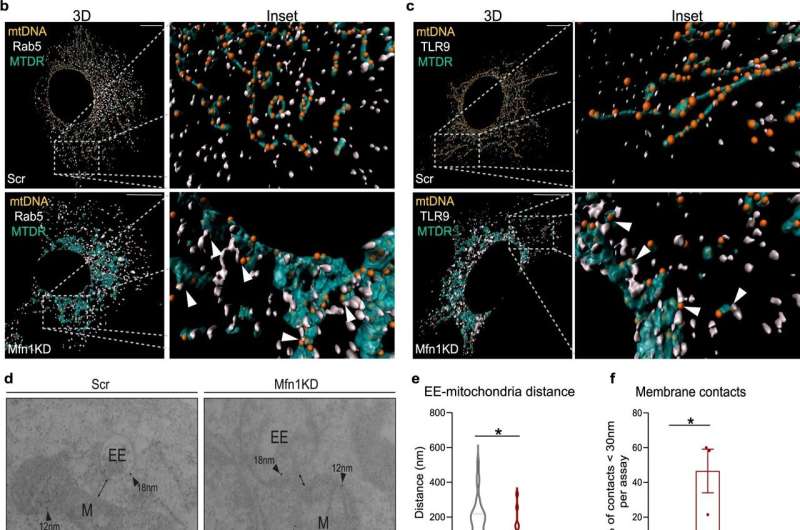
“One of the most remarkable findings of our study is that when we push mitochondrial dynamics towards either of its two extremes (mitochondrial fragmentation or elongation), these inflammatory pathways are activated in different ways. In both cases, the activation of these pathways involves the recognition of mitochondrial DNA by intracellular DNA sensors,” says Dr. Andrea Irazoki, the first author of the study, and a former Ph.D. student at IRB Barcelona who is currently working as a postdoctoral researcher at the University of Copenhagen.
The team of scientists has also validated that this mechanism has physiological relevance, as mice with mitochondrial fragmentation in their muscles display both inflammation and muscle atrophy, which negatively affects their physical performance.
Mitochondrial alterations and aging
Dr. David Sebastián, who has co-led the work together with Dr. Zorzano, says, “In this research we have discovered an essential role for mitochondrial dynamics in inflammatory processes. Together with the group’s previous results, which revealed alterations in dynamics during aging, these new findings could explain the increased inflammation associated with aging.”
Dr. Sebastián, an associate researcher at IRB Barcelona, is now a lecturer at the Faculty of Pharmacy and Food Sciences at the University of Barcelona and the Head of the Mitochondria, Metabolic Diseases and Aging laboratory. Last year, the same laboratory published another article that concerned the study of mitochondrial function, in which it was observed that problems in the elimination of damaged mitochondria also led to muscular atrophy and aging.
Dr. Zorzano’s laboratory works on the search for molecules that take over for certain dysfunctional mitochondrial proteins in order to guarantee cellular homeostasis. An increasing number of studies are underpinning the concept that mitochondrial alterations are the reason for a large number of diseases that involve inflammation.
More information:
Andrea Irazoki et al, Disruption of mitochondrial dynamics triggers muscle inflammation through interorganellar contacts and mitochondrial DNA mislocation, Nature Communications (2023). DOI: 10.1038/s41467-022-35732-1
Journal information:
Nature Communications
Source: Read Full Article


