Magrolimab plus azacitidine results in promising activity in higher-risk MDS patients
Myelodysplastic syndromes (MDS) are a diverse group of bone marrow disorders in which immature stem cells do not mature to become red blood cells, white blood cells or platelets. Patients with higher-risk MDS tend to have poorer outcomes, a greater chance of developing acute myeloid leukemia and few effective treatment options.
In a new article published today in the Journal of Clinical Oncology, Moffitt Cancer Center physician-scientists, in collaboration with institutions from across the United States and the United Kingdom, share promising phase 1b trial results of a new combination therapy—magrolimab + azacitidine—for patients with higher-risk MDS.
The prognosis of patients with MDS varies based on numerous factors, including genetic alterations and symptoms. Patients with higher-risk MDS have survival rates of less than two years and are commonly treated with more aggressive drugs called hypomethylating agents, such as azacitidine. While clinical trials of hypomethylating agents resulted in modest improvements in overall survival in higher-risk patients, response rates remain low (~40%), suggesting that improved treatment regimens are needed.
Magrolimab is a drug that targets the molecule CD47, which is found on most cancer cells, including the abnormal cells in MDS. CD47 inhibits immune cells called macrophages that engulf and destroy cancer cells or infectious microorganisms. By binding and targeting CD47, magrolimab blocks the “don’t eat me signal” on the MDS cells and activates immune cells to seek and kill cancer cells.
Preclinical studies have shown that the hypomethylating agent azacitidine cooperates with magrolimab to enhance this immune process via upregulation of the “pro-eat me” signal. Given these results, the team of researchers wanted to assess whether the combination of magrolimab and azacitidine would be effective and safe in patients with higher-risk MDS.
The researchers conducted a phase 1b clinical trial in 95 MDS patients with intermediate, high and very high-risk prognostic scores. The magrolimab plus azacitidine regimen was well tolerated. The most common adverse events were constipation (68.4%), thrombocytopenia or low blood platelet count (54.7%), anemia (51.6%), neutropenia or too few neutrophil white blood cells (47.4%), nausea (46.3%) and diarrhea (43.2%). A few patients had to undergo magrolimab dose reductions or discontinuations due to adverse events.
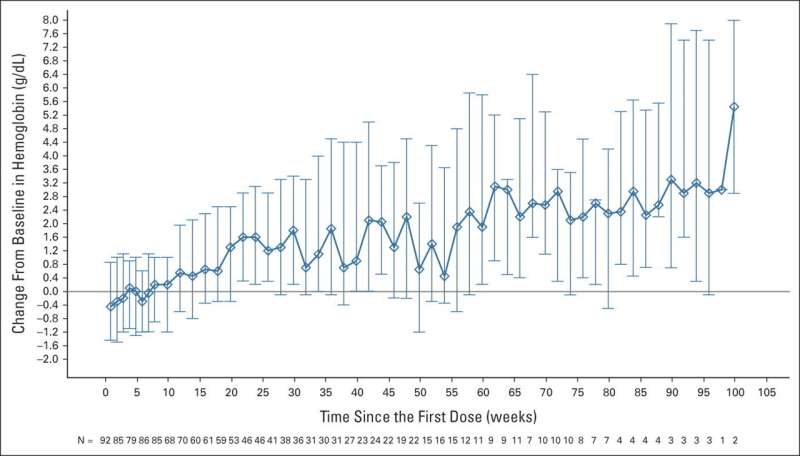
The magrolimab plus azacitidine regimen results are promising. Of patients who were evaluable, 33% achieved a complete response and 75% had either a complete or partial response to the therapy. Additionally, 40% of patients who had mutations in the TP53 gene, which often makes treatment more difficult, had a complete response to therapy.
Furthermore, with a 17.1-month median follow-up, the median overall survival rate was not yet reached in the study, which is encouraging considering many patients had poor prognostic risk factors. In the TP53 mutant group, the median overall survival was 16.3 months, which is an improvement given prior studies have uniformly shown a median survival of less than one year.
“This phase 1b study represents the largest, most comprehensive data set for magrolimab with azacitidine in myeloid malignancies,” said David Sallman, M.D., lead study author and associate member of the Malignant Hematology Department at Moffitt.
“These phase 1b trial results helped us launch a larger phase 3 randomized trial called ENHANCE evaluating magrolimab plus azacitidine versus placebo plus azacitidine. If our results are confirmed, this would become our new standard of care treatment approach for patients with high-risk MDS moving forward and we eagerly await the readout of this study.”
More information:
David A. Sallman et al, Magrolimab in Combination With Azacitidine in Patients With Higher-Risk Myelodysplastic Syndromes: Final Results of a Phase Ib Study, Journal of Clinical Oncology (2023). DOI: 10.1200/JCO.22.01794
Journal information:
Journal of Clinical Oncology
Source: Read Full Article


