Mucoactive drugs block SARS-CoV-2 infection
In a recent study published on the bioRxiv* preprint server, researchers evaluate the effects of mucociliary active molecules on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in vitro.

Study: Mucociliary Clearance Augmenting Drugs Block SARS-Cov-2 Replication in Human Airway Epithelial Cells. Image Credit: chanchai howharn / Shutterstock.com
Background
The continual emergence of SARS-CoV-2 variants has threatened the efficacy of current coronavirus disease 2019 (COVID-19) vaccines and therapeutic agents such as antiviral drugs and monoclonal antibodies, thus warranting the need for other treatment options.
SARS-CoV-2 infection destroys ciliated respiratory cells and disrupts mucociliary transport (MCT) functions. Altered MCT could prolong COVID-19 and increase the risk of secondary long-term complications due to dysregulated immune responses and pulmonary damage. Thus, MCT-augmenting drugs could improve airway epithelial barrier functions, reduce SARS-CoV-2 replication, and ultimately improve COVID-19 outcomes.
About the study
In the present study, researchers investigate whether augmenting or re-establishing MCT functions could improve COVID-19 outcomes.
The anti-SARS-CoV-2 activity of MCT-improving drugs, including ARINA-1, ivacaftor, camostat mesylate, poly-N (acetyl, arginyl) glucosamine (PAAG), allopurinol, ascorbic acid, reduced glutathione, sodium bicarbonate, N-acetylcysteine, high molecular weight hyaluronic acid (HA), BAPTA/AM, and sulforaphane, was tested in vitro.
To explore these agents' mechanistic and functional aspects, human bronchial epithelial cells (HBECs) were grown and differentiated in an air-liquid interface (ALI). Among the tested drugs, ARINA-1, the most effective, was investigated further using donor cell lines referred to as WT-128, WT-148, WT-152, WT-158, and WT-210.
Nasal epithelial cells were obtained from healthy and primary ciliary dyskinesia patients. In addition, the heterozygous pathogenic genotypes of both PCD patients were analyzed.
Viral loads were determined by reverse transcription quantitative-polymerase chain reaction (RT-qPCR) analysis. Infectious viruses were measured, and 50% tissue culture infectious doses (TCID50) were calculated.
The cytotoxicity of all mucociliary active molecules, except ARINA-1, was measured using non-radioactive cytotoxicity assays based on lactate dehydrogenase (LDH) release. ARINA-1 cytotoxicity was assessed using cell viability assays measuring intracellular adenosine triphosphate (ATP) levels.
Immunohistochemistry analysis was performed, and the length of cilia was measured.
Study findings
To some extent, all mucociliary active compounds inhibited SARS-CoV-2 proliferation in HBECs, among which camostat mesylate, ARINA-1, and HA conferred the greatest degree of protection. ARINA-1, which comprises ascorbate, glutathione, and bicarbonate, was the most effective compound, as demonstrated by the inhibition of damage to cells, improved MCT cellular responses, and intact ciliary motion, expression, and terminal differentiation.
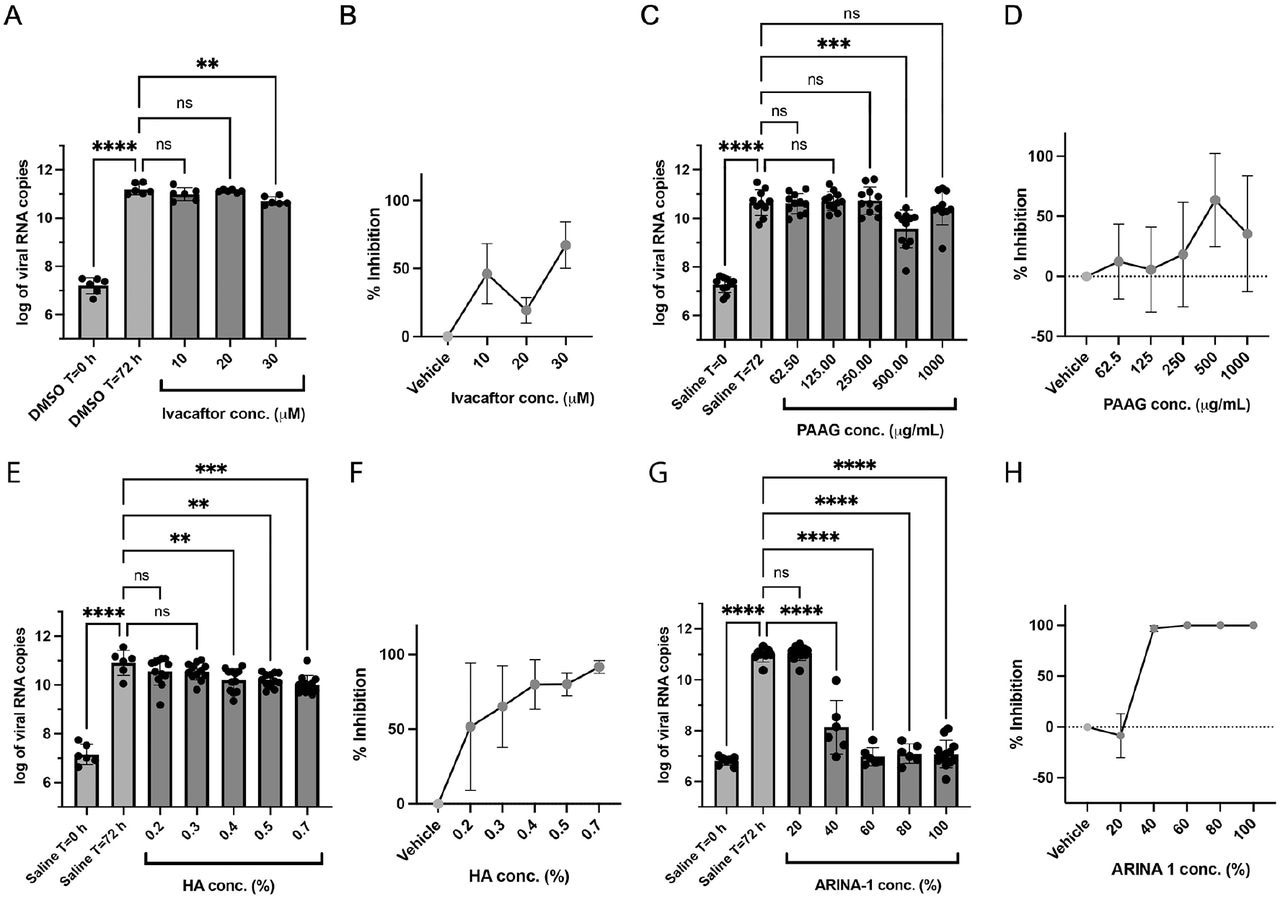
Ivacaftor moderately affected SARS-CoV-2 replication, with maximal efficacy at 30 µM, which is not likely to be of relevance in vivo. PAAG also demonstrated moderate activity against SARS-CoV-2; however, both PAAG and ivacaftor were cytotoxic when tested at concentrations needed for anti-SARS-CoV-2 effects.
HA showed greater than 90.0% SARS-CoV-2 inhibition at 0.70% concentration. Neither ARINA-1 nor HA caused cytotoxicity at the highest tested concentrations.
Drug Discovery eBook

Anti-SARS-CoV-2 activity of the mucoactive agents tested in well-differentiated primary HBE cells. A) Effect on the viral copy number measured by the RT-qPCR caused by ivacaftor at the concentrations shown on the graph. B) Data from graph in A) were converted to percent of viral inhibition. C-D), E-F) and G-H) As in (A-B), but using the compounds PAAG, HA and ARINA-1, respectively. DMSO was the vehicle used for ivacaftor (hydrophobic compound) and saline for PAAG, HA and ARINA-1 (hydrophilic compounds). All experiments were performed at least in duplicate independent assays, each with at least three transwell filter replicates per condition. Treatments were compared using ordinary one-way ANOVA statistical analysis. Hydrophobic compounds (added basolaterally) and hydrophilic (added apically) are shown in green and red, respectively. For each compound, each independent experiment was done with primary HBEC from a different donor.
Histopathological examinations showed that ARINA-1 inhibited SARS-CoV-2 shedding by primary HBECs and protected cells from SARS-CoV-2-mediated cytopathogenic effects. ARINA-1 induced hyper normal MCT in well-differentiated primary HBECs and prevented ciliary shortening.
ARINA-1 did not confer protection when ciliary beating was prevented using BAPTA/AM or when cilia were inactive or absent in the cells. SARS-CoV-2 ribonucleic acid (RNA) levels determined by RT-qPCR were in accordance with that of infectious SARS-CoV-2 in HBECs/air-liquid interface-based assays.
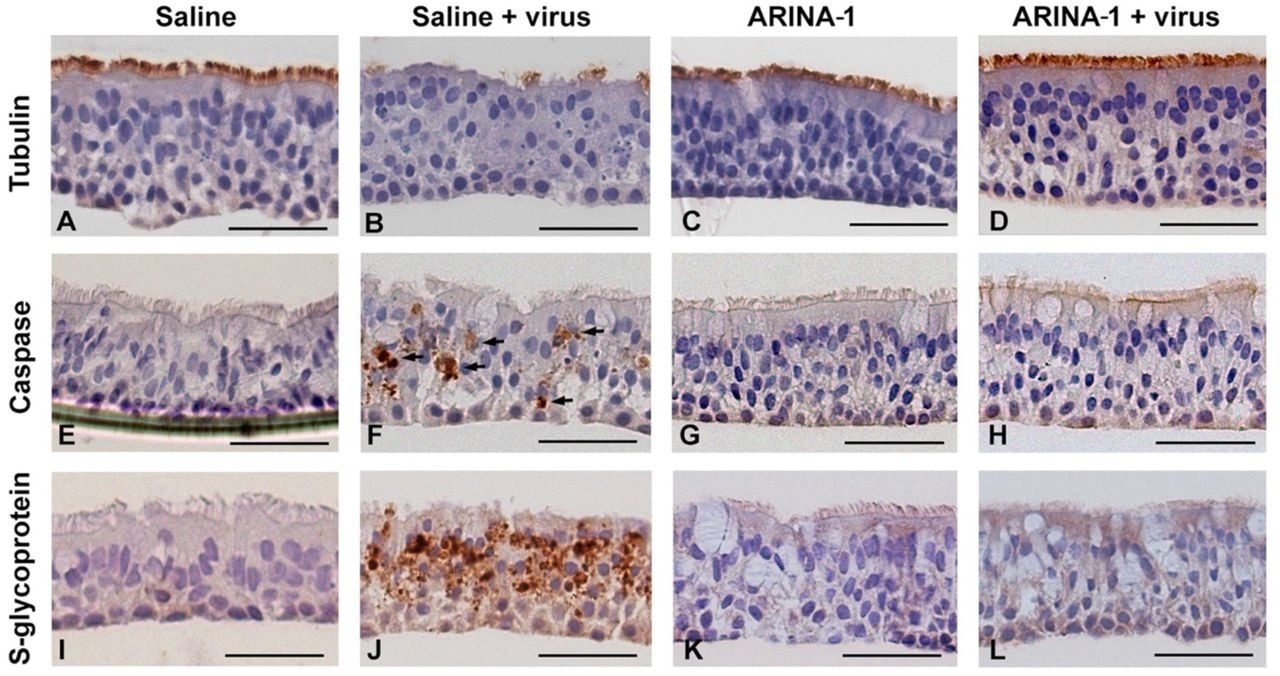
Histopathology studies showed that ARINA-1 protects primary HBE cells from SARS-CoV-2-mediated cytopathology. A-L) Representative photo micrographs of HBEC cross-sections with the immunohistochemistry and treatments. Each row corresponds to the immunohistochemistry using the antibody against the cell marker shown at the left, and each column corresponds to the treatment shown above the upper pictures. SARS-CoV-2 caused cilia shortening and loss in saline-treated cells (panel B). However, ARINA-1 protected cilia from damage (panel D). Similarly, the virus induced significant apoptosis in the mock-treated HBE cells (panel F, apoptotic cells indicated with arrows), which was not observed in those treated with ARINA-1 (panel H). In addition, mock-treated cells showed significant immunostaining using an antibody against the viral S glycoprotein (panel J), which again was not observed in the ARINA-1 treated cells (panel L). Scale bars represent 100 µm. Fully differentiated primary HBEC from two donors were used for the histopathology study. N β 10 pictures per donor and condition were taken and analyzed.
The authors hypothesized that SARS-CoV-2 infection increased reactive oxygen species (ROS) to levels that were injurious to ciliary movements, thereby preventing SARS-CoV-2 clearance and favoring SARS-CoV-2 attachment to the host. Thus, the antioxidant properties of ARINA-1 likely reduced ROS levels needed to neutralize the effect of SARS-CoV-2 on ciliary movements, which subsequently improved MCT.
Conclusions
Overall, the study findings showed that MCT augmentation or re-establishment could prevent SARS-CoV-2 infection and improve COVID-19 outcomes, thus supporting further investigation of MCT-enhancing drugs.
*Important notice
bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Campos-Gomez, J., Petty, C. F., Mazur, M., et al. (2023). Mucociliary Clearance Augmenting Drugs Block SARS-Cov-2 Replication in Human Airway Epithelial Cells. bioRxiv. doi:10.1101/2023.01.30.526308. https://www.biorxiv.org/content/10.1101/2023.01.30.526308v1
Posted in: Drug Discovery & Pharmaceuticals | Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Adenosine, Adenosine Triphosphate, Allopurinol, Antibodies, Antibody, Antioxidant, Apoptosis, Ascorbic Acid, Cell, Cilia, Compound, Coronavirus, Coronavirus Disease COVID-19, Cytotoxicity, Drug Discovery, Drugs, Dyskinesia, Efficacy, Enhancing Drugs, Glucosamine, Glycoprotein, Histopathology, Immunohistochemistry, in vitro, in vivo, Intracellular, Oxygen, Polymerase, Polymerase Chain Reaction, Primary Ciliary Dyskinesia, Proliferation, Respiratory, Ribonucleic Acid, RNA, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Sodium Bicarbonate, Syndrome, Tissue Culture, Transcription, Virus

Written by
Pooja Toshniwal Paharia
Dr. based clinical-radiological diagnosis and management of oral lesions and conditions and associated maxillofacial disorders.
Source: Read Full Article


