Neuronal sensing of cytokine IL-12 induces tissue adaptation and protects mice from neuroinflammation
Cytokines are proteins that help the human body to control and support the work of the immune system. Over the past few decades, a growing number of studies have been exploring the role of specific cytokines, including IL-12, in inflammation and the development of specific diseases, such as psoriasiform skin inflammation and multiple sclerosis (MS).
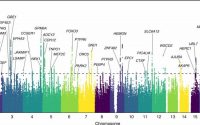
Researchers at the University of Zurich in collaboration with other research institutes recently set out to map the IL-12 receptor expression on different type of cells, including natural killer (NK) and T cells but also, surprisingly, neurons and oligodendrocytes. Their paper, published in Nature Neuroscience, gathered interesting new insights about the mechanisms underlying the impact of these proteins on inflammation and the development of MS.
“The two cytokines IL-12 and IL-23 share a common subunit: the protein p40,” Prof. Burkhard Becher, co-senior author of the paper, told Medical Xpress. “For a long time, researchers interpreted findings with p40 neutralization as an effect of both IL-12 and IL-23. Already in the early 2000s, during my postdoc in the U.S., I serendipitously discovered that IL-23, rather than IL-12, had proinflammatory, pathogenic properties in CNS neuroinflammation Specifically, my colleagues and I noticed that IL-12 has a regulatory function and that it limits inflammation.”
In the studies he conducted as a post-graduate, Prof. Becher gathered initial evidence suggesting that that IL-12 played a key role in attenuating inflammation. At the time, however, due to a lack of suitable genetic tools, he was unable to uncover the specific mechanisms through which IL-12 protected the body from inflammation.
“In 2016, Dr. Sarah Mundt joined my lab as a postdoc to generate this missing tool,” Prof. Becher said. “The most pressing question was now which cell type could be responsible for the observed effect. While I was convinced that the IL-12 sensing cell population must lie within the hematopoietic compartment, Dr. Mundt and Dr. Myrto Andreadou, who started as a Ph.D. around the same time, were curious to see whether also other cell types, possibly within the central nervous system (CNS) itself, could be the mediators of the protective effect of IL-12.”
The primary objective of this research team’s recent study was to shed further light on the role that the IL-12 cytokine plays in inflammation. To do this, they conducted a series of experiments in adult mice, using a genetic tool developed by Drs. Mundt and Andreadou.
“Cytokines are a way of immune cells to communicate with each other and with other cells of the body,” Dr. Mundt, co-senior author of the paper, explained. “To deliver their ‘message,’ cytokines must engage with their corresponding receptor on the surface of cells. In our study, we used a newly developed genetically modified mouse model that allowed us to identify IL-12 receptor expressing cells and to specifically ablate the IL-12 receptor from individual cell types.”
In their experiments, Drs. Becher, Mundt, Andreadou and their colleagues targeted the IL-12 receptor in neurons within the adult mice’s brains, removing it using a technique known as conditional gene ablation. Subsequently, they observed the effects of its removal on the clinical disease course in a mouse model of MS.
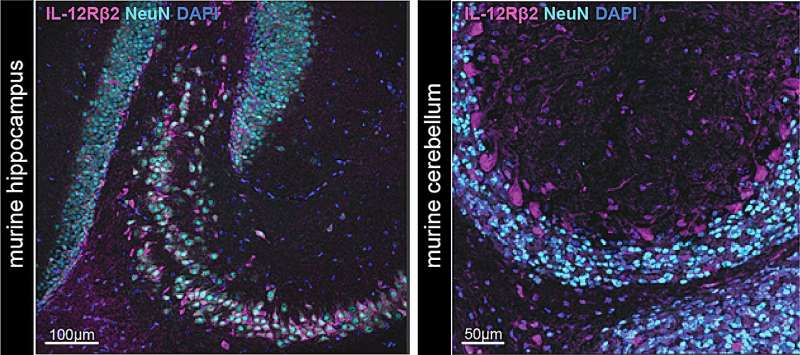
“We found that neurons expressed high levels of the IL-12 receptor and potentially represented the long missing mechanistic link,” Dr. Andreadou said. “By specifically ablating the receptor in neurons we could recapitulate Prof. Becher’s old finding that IL-12 ameliorated the clinical outcome in a murine model of MS. Thereby, we identified neurons as the responsible cell type that mediated the protective effect of IL-12.”
Finally, the researchers tried to decode the “message” that IL-12 had delivered to neurons, using an advanced genetic technique known as single nucleus RNA sequencing. Interestingly, they found that the cytokine had instructed the neurons to protect themselves and their surrounding environment, via their immune responses.
“Our findings explain why previous attempts in targeting IL-12 in MS therapy has not been successful,” Prof. Becher said. “We can now say with confidence that such a therapeutic approach would even be counterproductive, and, conversely, delivering IL-12 induced ‘messages’ (such as trophic factors) might be beneficial in MS.”
The recent work by this research team gathered valuable insight that could guide the development of future development of treatments for MS and potentially also other diseases linked to inflammation. Their findings are aligned with other recent works in this area, as some clinical trials have already been assessing the potential of increasing neurotrophins, such as BNDF (which the team found to be induced by IL-12) as a part of treatment for MS.
“Beyond that, this discovery sheds further light on the multifaceted properties of cytokines,” Drs. Becher, Mundt and Andreadou said. “We now find that all cytokines may have simultaneously both pro- and anti-inflammatory effects depending on their cellular targets. This makes a lot of sense in order to balance immune responses, which should be effective, albeit self-limiting to avoid immune-mediated pathology and chronic inflammation.”
This recent study could soon pave the way for further research efforts aimed at exploring the impact of cytokines in neuroinflammation and associated diseases using genetics tools. Meanwhile, the researchers will continue their work investigating IL-12 in mouse models of MS and other debilitating diseases of the CNS, including Alzheimer’s disease (AD).
“We are currently collaborating with the team of Prof. Frank Heppner in Germany to study the role of IL-12 in a model of AD, a neurodegenerative disorder of the CNS,” Prof. Becher added. “In this instance, the cells that deliver IL-12 to the CNS differ from those in neuroinflammatory conditions like MS. Hence, the induced message could also be different.”
More information:
Myrto Andreadou et al, IL-12 sensing in neurons induces neuroprotective CNS tissue adaptation and attenuates neuroinflammation in mice, Nature Neuroscience (2023). DOI: 10.1038/s41593-023-01435-z
Journal information:
Nature Neuroscience
Source: Read Full Article