New directions for HIV treatment
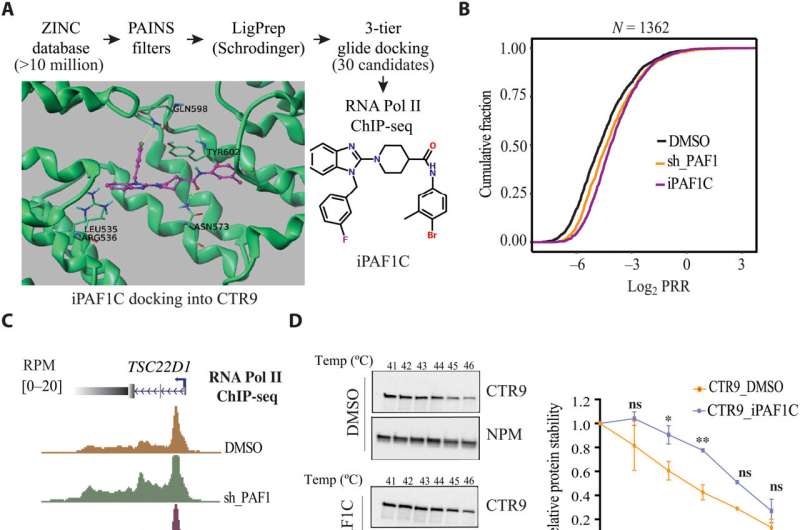
Northwestern Medicine scientists have identified a new compound which could inform future HIV cure strategies, according to research published in Science Advances.
The current standard treatment for people living with HIV is antiretroviral medication, which suppresses viral replication and spread, but does not cure HIV. This is because the virus can hide inside immune cells, making itself undetectable to the body’s immune system and resistant to antiretroviral therapy.
The newly identified compound works to boost an experimental approach, dubbed “shock and kill,” during which the latent virus is activated so it can be targeted by the body’s immune system or antiretroviral drugs.
In the study, investigators utilized computer modeling to identify a compound which inhibits the Polymerase Associated Factor 1 Complex (PAF1C), which had been found to play a role in repressing viral gene expression in a previously published Northwestern Medicine study.
The study authors discovered that the compound, which they named iPAF1C, enhanced the activity of HIV latency reversal drugs in cell culture.
The scientists also tested the effects of iPAF1C on immune cells taken from the blood of four people living with HIV. They found that while the compound alone could activate HIV transcription, it had the greatest effect when administered alongside other latency-reversal drugs.
The compound is a promising new direction for HIV treatment, said Ali Shilatifard, Ph.D., the Robert Francis Furchgott Professor and chair of Biochemistry and Molecular Genetics, and senior author of the study.
“With this compound, we have something that can regulate RNA Polymerase II pause-release sites in these cells, so we can biologically study the role of this pause-release regulated by PAF1 in different cells,” said Shilatifard, who is also a member of Robert H. Lurie Comprehensive Cancer Center of Northwestern University and Leader of the Center’s Cancer Epigenetics & Nuclear Dynamics Program. “This is something that could potentially have great clinical application.”
Judd Hultquist, Ph.D., assistant professor of Medicine in the Division of Infectious Diseases and a co-author of the study, said the research was a result of teamwork between departments.
“This study is a great example of inter-institutional collaboration,” Hultquist said. “Ali brought up the idea of utilizing small molecules that he and postdoc Shimaa have explored in the context of cancer in some of our HIV assays. With support from the Third Coast Center for Aids Research, we were able to formalize this collaboration and examine these protein complexes in the context of HIV replication, and the results turned out to be very interesting.”
The discovery also adds to the understanding of HIV latency, Hultquist said.
“The fact that this compound can reactivate latent HIV infection is exciting because there hasn’t been a compound like this that has made it to the clinic, so it could represent a new strategy toward latency reversal,” said William Cisneros, a fourth-year Ph.D. student in the Driskill Graduate Program in Life Sciences and a co-author of the study.
Future research will focus on refining the compound for testing in animal models, the study authors said.
“We are working on further development and identification of more iPAF1C compounds to get a more efficient small molecular inhibitors of pause release, with the goal of eventually getting a clinical use compound,” said Shimaa Soliman, Ph.D., a postdoctoral research fellow in the Shilatifard lab and first author of the study. “Moreover, we are testing the potential of iPAF1C in treating other diseases in which PAF1 plays an oncogenic role, such as in cancer.”
More information:
Shimaa H. A. Soliman et al, Enhancing HIV-1 latency reversal through regulating the elongating RNA Pol II pause-release by a small-molecule disruptor of PAF1C, Science Advances (2023). DOI: 10.1126/sciadv.adf2468
Journal information:
Science Advances
Source: Read Full Article


