New method used to develop RNA therapy for the treatment of rare diseases
Having a rare genetic disease is actually pretty common. Rare diseases affect approximately 1 in 10 individuals, and more than 30 million people in the U.S. have a rare disease diagnosis. What makes them rare is that these 1 in 10 people affected have an estimated 7,000 different conditions, with treatments available for only about 5% of them.
Rare disease research led by the Korea Advanced Institute of Science and Technology (KAIST), Republic of Korea, has accelerated the treatment potential for one such disease, ataxia-telangiectasia, with antisense oligonucleotides.
In a paper, “A framework for individualized splice-switching oligonucleotide therapy,” published in Nature, the team details their methods to identify treatment potential for one rare disease and illustrate how the process could tackle other untreatable conditions. A Clinical Briefing published in the same journal issue summarizes the work done by the team.
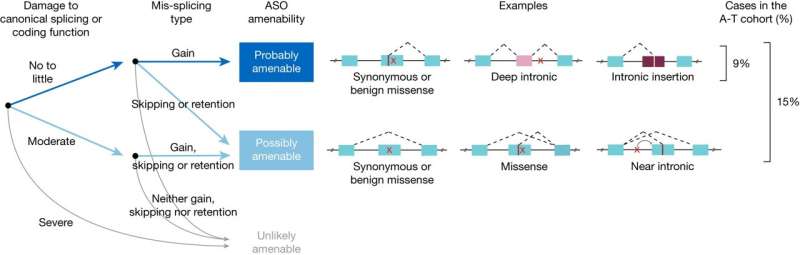
The research is based on splice-switching antisense oligonucleotides (ASOs). ASOs are short sequences of synthetic nucleic acids, a chain of nucleotides adenine (A), cytosine (C), guanine (G) and thymine (T), connected in a specific sequence. The specific sequence is called “antisense” because it complements an RNA target sequence, binding with it to alter its function.
The alteration can induce degradation, modulation of splicing, prevention of translation, or in this case, paste a correction over a mis-splicing event. By correcting mis-spliced RNA, the normal production of downstream proteins can resume the role they would carry out in a healthy individual.
Ataxia-telangiectasia (A-T) is caused by a loss of function of ATM, a gene involved in the cellular response to DNA double-strand breaks. A-T is characterized by progressive cerebellar degeneration, immunodeficiency and high cancer susceptibility. Early manifestations include ataxia, involuntary movements, neuropathy, oculomotor apraxia, dysphagia, slurred speech and ocular and skin telangiectasias.
The disease has a poorly understood prevalence, with estimates as high as 1 in 40,000 or as low as 1 in 100,000 live births worldwide. Average life expectancy is low at just 25 years, and death is most frequently due to lung disease or cancer.
The researchers performed whole-genome sequencing on 235 individuals with A-T to develop a predictive taxonomy assessment of variants targetable by ASO intervention. ASOs were then designed and tested successfully in patient tissue samples, rescuing the mis-spliced mRNA and restoring ATM cell signaling.
In a pilot clinical study, one of the ASOs created was administered to a child with A-T and showed good tolerability for three years. The study provides a framework for prospectively identifying individuals who may benefit from splice-switching ASO therapy.
Rare diseases like A-T often lack effective therapies because they require an intensive understanding of a single patient’s genetic variants and custom-made therapeutics. Health care systems are designed around more universally understood treatments and pathologies, leaving 10% of the population with rare disease diagnoses untreated.
Over a hundred ASOs are in various stages of clinical trials, and hundreds more are being developed. The treatment potentials range from rare diseases not covered in medical school to more well-studied afflictions like Alzheimer’s disease, Parkinson’s disease, and rheumatoid arthritis. ASOs also have tremendous potential in most forms of cancer.
The current study presents a framework for identifying individuals with genetic variants treatable by an ASO therapeutic approach, accelerating the development of custom-made ASOs for a wide range of neglected diseases.
From completely untreatable to simply untreated
One of the obstacles ASOs face is the current cost of treatment. FDA-approved ASO treatments can cost hundreds of thousands of dollars a year for a single individual, and many national health care systems and insurers are declining patient access to treatment.
This is likely a temporary situation that could get much worse before it improves. It could worsen in that more ASO treatments will become approved and available to the public only to be denied access for cost reasons. As with any drug platform in the past, this should improve as demand rises and ASOs become a streamlined part of therapeutic manufacturing processes.
If governments and health care systems are not prepared to incur the current costs of treatments, investment in research and manufacturing technology that will optimize production and lower costs in the future should be a top priority.
ASOs are poised to improve the lives of hundreds of millions of people across multiple diseases and disorders, reducing mortality and eliminating the negative effects of a wide range of neurodegenerative diseases. Not being prepared to deliver these treatments would be a major global health crisis.
More information:
Jinkuk Kim et al, A framework for individualized splice-switching oligonucleotide therapy, Nature (2023). DOI: 10.1038/s41586-023-06277-0
Clinical Briefing: A framework for identifying targets for individualized therapy in genetic disease, Nature (2023). DOI: 10.1038/d41586-023-01994-y
Journal information:
Nature
Source: Read Full Article


