Real-world data on the effectiveness of Sotrovimab as a prophylactic against COVID-19

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
In a recent study posted in the medRxiv* preprint server, scientists assessed the efficacy of sotrovimab for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) treatment.
Emerging SARS-CoV-2 variants have lowered the fold change in half maximal effective concentration (EC50) for the SARS-CoV-2 Omicron BA.2 sublineage and subsequent sublineages. Yet, the association between this decrease and clinical efficacy outcomes is unknown. With a lack of clinical trials evaluating the efficacy of sotrovimab against novel variants, real-world evidence becomes an essential data source.
 Study: Real-world effectiveness of sotrovimab for the treatment of SARS-CoV-2 infection during Omicron BA.2 subvariant predominance: a systematic literature review. Image Credit: Cryptographer / Shutterstock
Study: Real-world effectiveness of sotrovimab for the treatment of SARS-CoV-2 infection during Omicron BA.2 subvariant predominance: a systematic literature review. Image Credit: Cryptographer / Shutterstock
About the study
In the present study, researchers assessed the efficacy of sotrovimab on severe coronavirus disease 2019 (COVID-19) outcomes throughout the period of the prevalence of the SARS-CoV-2 Omicron BA.2 subvariant.
This systematic literature review (SLR) comprised observational papers assessing clinical outcomes as well as the viral load in sotrovimab-treated patients, which were published between 1 January 2022 and 3 November 2022 in preprint articles, peer-reviewed journal publications, and conference abstracts. To identify data related to Omicron BA.2 and the following subvariants, the team chose a suitable publication period for the systematic review.
The following electronic databases were searched on 3 November 2022: MEDLINE, LitCovid, Embase, EcoLit, and Cochrane COVID-19 Study Registry. Further searches were undertaken in medRvix, bioRvix, arRvix, xhemRvix, Preprints.org, SSRN, and ResearchSquare for relevant preprints. In addition, relevant abstracts from the following conferences were indexed beginning in January 2022: Infectious Diseases Week, International Conference on Emerging Infectious Diseases, European Respiratory Society, and European Congress of Clinical Microbiology and Infectious Diseases.
Data extraction from the listed studies was conducted by a single extractor using a Microsoft Excel-designed data extraction file. Information extracted included the study's title and citation, data source, study design and details, country, number of patients, study population, data collection period and circulating SARS-CoV-2 variants, duration of follow-up, key baseline features, and clinical outcomes. The clinical outcomes taken into account for the study included hospital admission, intensive care admission, respiratory support, emergency department visits, mortality, COVID-19 progression, the relative and absolute change in viral load observed during the acute phase after sotrovimab therapy, and the number of patients having undetectable viral load after sotrovimab treatment.
Results
Initial searches of electronic databases generated 257 studies. Another 263 studies were found by searching preprints, conference abstracts, and citation chasing from appropriate SLRs. After removing duplicates, 343 unique abstracts and titles were evaluated, of which 89 were deemed eligible for full-text review. Five observational trials reporting viral load or clinical outcome data associated with sotrovimab during the era of BA.2 predominance were deemed appropriate for inclusion in the present SLR.
Drug Discovery eBook

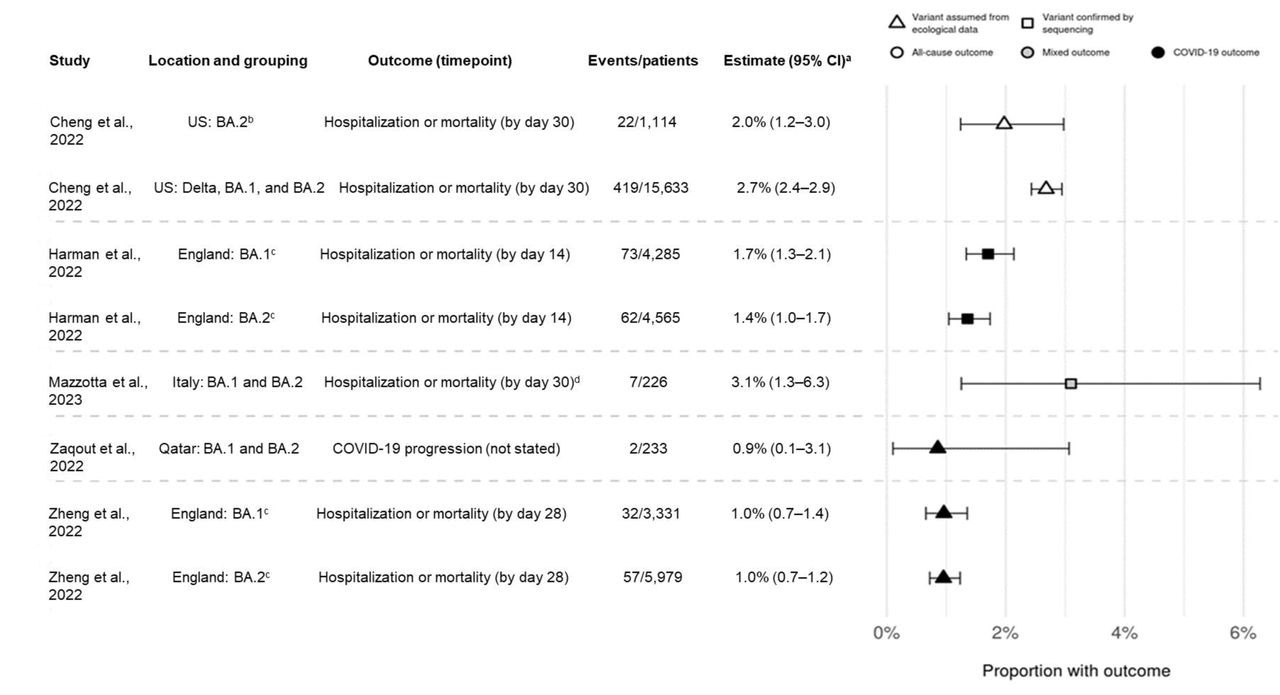
Point estimates for hospitalization or mortality (as a composite endpoint) or clinical progression for sotrovimab-treated patients. a95 CIs calculated via Clopper-Pearson methods using reported data. bDefined as March through April 2022 in source and assumes homogeneity in the distribution of SARS-CoV-2 variants across all US states. cOnly COVID-19-specific outcome shown; all-cause outcome also reported in source. dHospitalizations were COVID-19-specific; deaths could be due to any cause. CI confidence interval
The number of patients reporting hospitalization or fatality due to COVID-19 was consistently low for all investigations and periods of the prevalence of Omicron BA.1 and BA.2 variants. COVID-19-related hospital admission or mortality rates were between 1.0% and 3.1% for sotrovimab-treated patients during Omicron BA.1 prevalence and from 1.0% and 3.6% when BA.2 was predominant. The number of patients who reported hospitalization and mortality due to all causes ranged from 2.1% to 2.7% for the BA.1 predominance era, and from 1.7% to 2.0% for the BA.2 era. During Omicron BA.1 predominance, COVID-19-related mortality was projected to be 0.21% for the sotrovimab group versus 0.67% for the molnupiravir group, and 0.15% versus 0.96% for the BA.2 era, respectively.
During the BA.1 and BA.2 subvariant surges, sotrovimab was associated with a significantly decreased incidence of 28-day SARS-CoV-2-related hospital admission or fatality compared to molnupiravir. After statistical adjustment for demographics, vaccination status, high-risk cohort categories, body mass index, calendar time, and other comorbidities, the findings indicated that sotrovimab was associated with a significantly lower risk of COVID-19-related hospital admission or mortality compared to molnupiravir during the BA.1 and BA.2 periods.
During the BA.2 subvariant surge, sotrovimab was linked with a decreased risk of 30-day hospitalization or mortality from all causes compared to no mAb treatment. In March 2022, sotrovimab was considerably more successful than non-mAb-treated patients, with an adjusted reduction of 59% in relative risk and a propensity score-matched relative risk reduction of 64% with respect to 30-day all-cause hospital admission or mortality. Similar risks of hospitalization were associated with BA.1 and BA.2 patients treated with sotrovimab.
Conclusion
The study findings showed that sotrovimab continued to be clinically effective in mitigating severe clinical outcomes associated with SARS-CoV-2 infections during the period of SARS-CoV-2 Omicron BA.2 predominance compared to the control/comparator and relative to Omicron BA.1 predominance. During Omicron BA.1 and BA.2 subvariant predominance, the studies consistently reported low rates of poor clinical outcomes in individuals treated with sotrovimab.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Preliminary scientific report. Real-world effectiveness of sotrovimab for the treatment of SARS-CoV-2 infection during Omicron BA.2 subvariant predominance: a systematic literature review, Myriam Drysdale, Daniel C. Gibbons, Moushmi Singh, Catherine Rolland, Louis Lavoie, Andrew Skingsley, Emily J. Lloyd, medRxiv 2023.03.09.23287034, DOI: https://doi.org/10.1101/2023.03.09.23287034, https://www.medrxiv.org/content/10.1101/2023.03.09.23287034v1
Posted in: Medical Research News | Disease/Infection News | Pharmaceutical News
Tags: Body Mass Index, Coronavirus, Coronavirus Disease COVID-19, Efficacy, Hospital, Infectious Diseases, Intensive Care, Microbiology, Mortality, Omicron, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Sotrovimab, Syndrome

Written by
Bhavana Kunkalikar
Bhavana Kunkalikar is a medical writer based in Goa, India. Her academic background is in Pharmaceutical sciences and she holds a Bachelor's degree in Pharmacy. Her educational background allowed her to foster an interest in anatomical and physiological sciences. Her college project work based on ‘The manifestations and causes of sickle cell anemia’ formed the stepping stone to a life-long fascination with human pathophysiology.
Source: Read Full Article


