T cells require healthy mitochondria, research shows
All cells have their own power plants, called mitochondria. There are often more than 100 mitochondria per cell and each possesses their own genome, which in turn contains genes responsible for energy production. If errors creep into these genes, this can cause problems in the cell and result in diseases.
Scientists from the Berlin Institute of Health at Charité (BIH) and the Max Delbrück Center have now discovered that the T cells of the immune system are especially sensitive to genetic disturbances within their mitochondrial power plants. They have published their findings in the journal Nature Genetics.
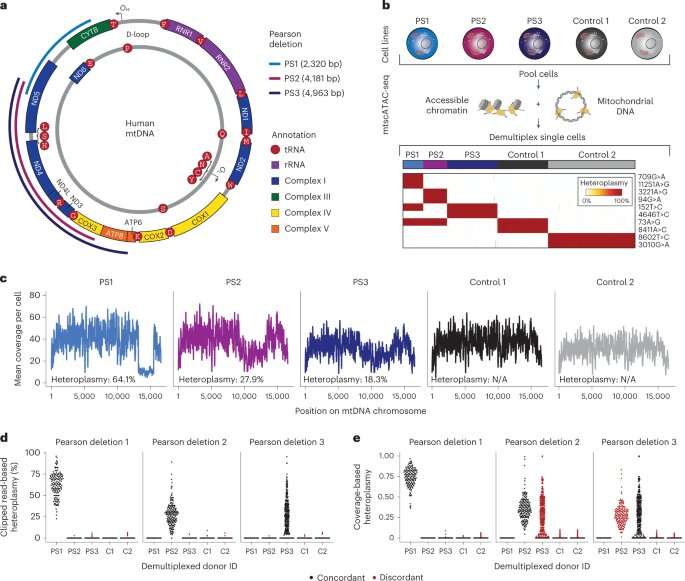
Patients with Pearson syndrome suffer from anemia because their bone marrow produces too few red blood cells. Immune system defects are also suspected, but these have not yet been studied in detail. The source of these problems are errors in the genome of the cellular power plants, the mitochondria, explains Dr. Leif S. Ludwig, head of the Emmy Noether Independent Junior Research Group “Stem Cell Dynamics and Mitochondrial Genomics” at the BIH and Max Delbrück Center.
“The genome of the mitochondria has large gaps (deletions) in these patients, which results in the cells not having enough energy to perform their various functions.”
No mutations in the mitochondria of some T cells
Ludwig’s group is part of the joint focus area “Single-Cell Approaches for Personalized Medicine,” which the BIH at Charité founded together with the Max Delbrück Center and Charité—Universitätsmedizin Berlin. The scientists are specialized in analyzing individual cells and were thus able to closely examine the patients’ blood and immune cells.
“Our research showed that the pathogenic changes in the mitochondrial genome were not equally present in all cells,” explains Ludwig, a cell biologist. “For example, the mitochondria in certain types of T cells were almost completely free of mutations. That was quite surprising.”
An explanation for this finding, according to Ludwig, is that when T cells are activated, they rely on the mitochondria to supply the energy needed for their continued maturation. “During a defense response, T cells need to proliferate substantially, and we think that especially these initial cell divisions don’t work properly without healthy mitochondria.”
Selection at play
Yet interestingly, different types of T cells show different degrees of tolerance to defects in the mitochondrial genome. Pathological mutations are frequently found in memory CD4+ T cells, but rarely in memory CD8+ T cells. “The way we explain this is that CD8+ T cells use the mitochondria differently,” says Ludwig.
“Since they require mitochondria that are completely healthy, we only see memory CD8+ T cells without mutations. Cells with ‘sick’ mitochondria are culled out or, as we cell biologists say, negatively selected.” As this is highly relevant for patients with mitochondrial disease, the scientists now want to do further research to see exactly how the mitochondria of different cells differ.
Ludwig, whose group is based at the Berlin Institute for Medical Systems Biology of the Max Delbrück Center (MDC-BIMSB), is translating his findings into medical applications in collaboration with his clinical partners at Charité, including Prof. Lars Bullinger and Prof. Ulrich Keller, the Directors of the Department of Hematology, Oncology and Tumor Immunology at Charité Campus Virchow-Klinikum (CVK) and Charité Campus Benjamin Franklin (CBF), respectively.
“We don’t yet know how therapeutically effective the use of base editing technologies—or even the transplantation of healthy mitochondria—will one day be at addressing changes in the mitochondrial genome,” says Ludwig, “but we are giving it serious thought.”
More information:
Caleb A. Lareau et al, Single-cell multi-omics of mitochondrial DNA disorders reveals dynamics of purifying selection across human immune cells, Nature Genetics (2023). DOI: 10.1038/s41588-023-01433-8
Journal information:
Nature Genetics
Source: Read Full Article


