Tuberculosis infection protects mice against COVID-19
Coronavirus disease 2019 (COVID19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2 or CoV2) and tuberculosis (TB) caused by Mycobacterium tuberculosis (Mtb) – are two fatal global respiratory infections with high mortality rates. Both these infections pose a significant health challenge and economic burden worldwide.
Although concurrent Mtb/CoV2 infection can exacerbate the symptoms of TB, the COVID 19 mortality rate among individuals with a previous Mtb infection appears to be lower. This could be due to a similarity between CoV2 antigens and proteins expressed by the Mycobacterium spp.
Thus, a prior TB infection will likely confer heterologous immunity against SARS-CoV-2.
 Study: Mice infected with Mycobacterium tuberculosis are resistant to acute disease caused by secondary infection with SARS-CoV-2. Image Credit: Kateryna Kon
Study: Mice infected with Mycobacterium tuberculosis are resistant to acute disease caused by secondary infection with SARS-CoV-2. Image Credit: Kateryna Kon
The study
A recent study published in the journal PLOS Pathogens evaluated the impact of Mtb infection on secondary CoV2 infection.
This study entailed the application of two COVID-19 mouse models (using chronically infected Mtb mice) – SARS-CoV-2 infection of K18-hACE2 mice and mouse-adapted SARS-CoV-2 infection of C57BL/6 mice.
Results
To study if prior Mtb infection affects the response to CoV2 infection, K18-hACE2 (ACE2) and C57BL/6 (B6) mice were infected with Mtb, and post thirty days, were exposed to CoV2.
Here, three groups of mice were monitored – ACE 2 mice with simultaneous Mtb/CoV2 infection (MtbPOS CoV2POS); sterile media challenged ACE2 mice with prior Mtb infection (MtbPOS CoV2NEG) – control group; and CoV2 challenged ACE2 mice without previous Mtb infection (MtbNEG CoV2POS – another control group. The mice were euthanized and studied 4, 7, and 14 days post-challenge.
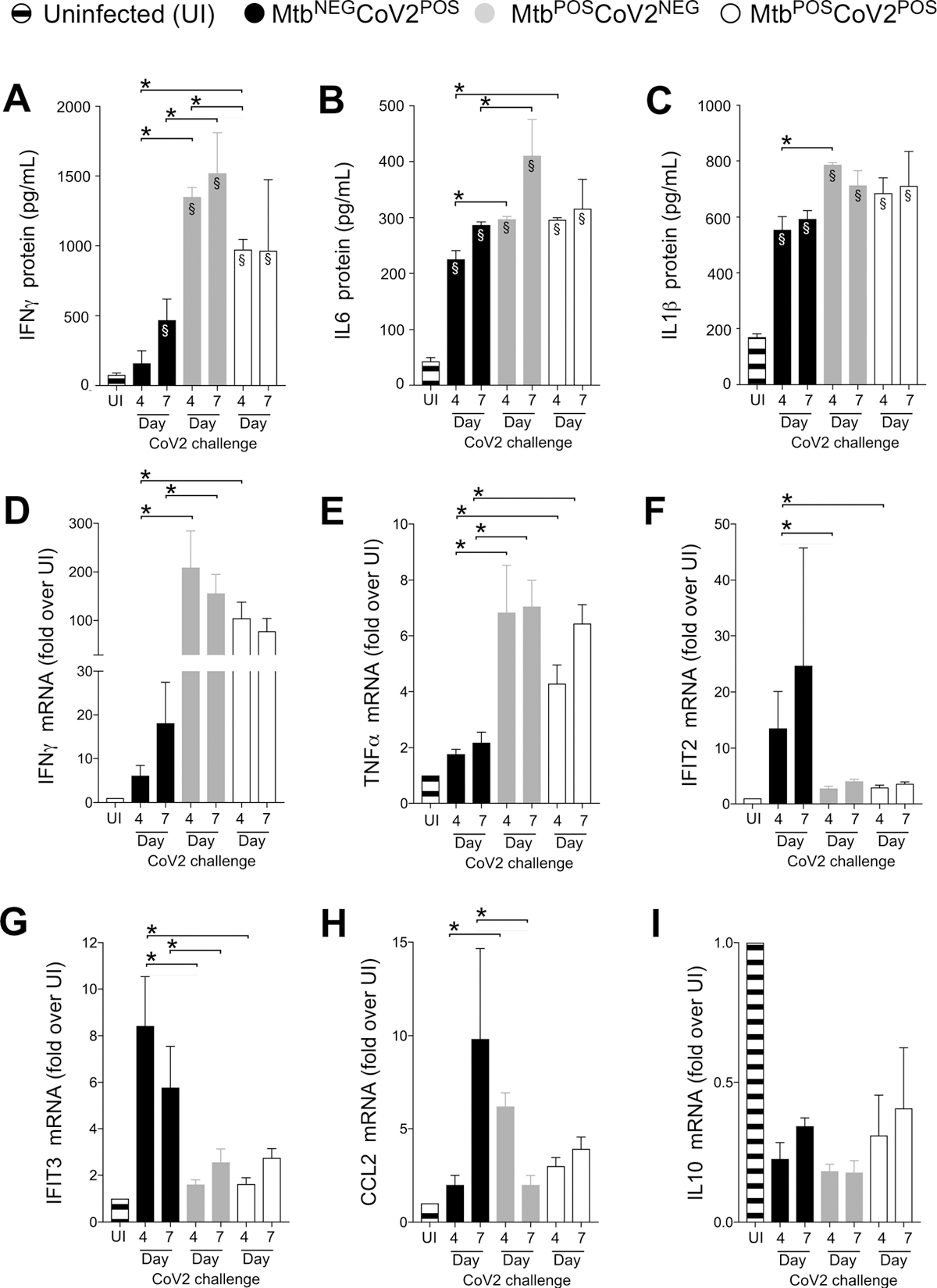
CoV2-elicted cytokine responses are muted in the presence of Mtb infection. On the indicated days, lung tissue from MtbNEGCoV2POS, MtbPOSCoV2NEG, MtbPOSCoV2POS and uninfected (UI) ACE2 mice was used to measure (A-C) protein levels of (A) IFNγ, (B) IL6 and (C) IL1β, as well as (D-I) mRNA levels of (D) IFNγ, (E) TNFα, (F) IFIT2, (G) IFIT3, (H) CCL2 and (I) IL10. This experiment was repeated twice, each with similar results (4 mice/group/timepoint). *, p ≤ 0.05 as determined by either Student’s t-test or ANOVA; §, significant relative to UI protein levels.
On day-7, the MtbNEG CoV2POS control lost significant weight, while weight loss was insignificant in MtbPOS CoV2POS ACE2 mice—which were indistinguishable from the MtbPOS CoV2NEG ACE2 mice.
On the 4th day post-challenge, CoV2 burden in the lungs was found to be lower in the MtbPOS CoV2POS group compared to the MtbNEG CoV2POS control. The lungs, liver, and spleen of the MtbPOS CoV2POS group and the MtbPOS CoV2NEG control were similar post-challenge – suggesting that they remained unaffected by Mtb growth post-CoV2 infection.
The number of acid-fast bacilli (AFB) in the lungs was comparable in the MtbPOS CoV2POS group and the MtbPOS CoV2NEG control. The colony-forming unit (CFU) burden in Mtb-infected B6 controls was comparable to the MtbPOS CoV2NEG control indicating an unaffected Mtb growth by the expression of transgenic human ACE2.
An assessment of CoV2 induced immune response post-Mtb infection was done. Multiple inflammatory genes were expressed in the lungs post-CoV2 infection. On days 4 and 7 post-challenge, the levels of interleukin (IL)1b, IL6, and interferon (IFN)a were elevated in the MtbNEG CoV2POS lungs compared to those in the uninfected controls; the levels were even higher for the MtbPOS CoV2NEG control.
Meanwhile, MtbPOS CoV2POS resistance could not be associated with higher levels of antiviral genes (IFIT2 and IFIT3), which were expressed in MtbNEG CoV2POS controls. Concurrent Mtb/CoV2 infection did not induce CCL2 expression.
Of note, on days 4 and 7 post-challenge, MtbNEG CoV2POS controls exhibited alveolar necrosis and diffuse alveolar damage. Terminal bronchiole pneumonia and formation of the hyaline membrane were noted in MtbNEG CoV2POS controls, which were absent in MtbPOS CoV2POS lungs. Individual size and the cumulative area occupied by TB granules were comparable between MtbPOS CoV2POS and MtbPOS CoV2NEG lungs. In addition, immunohistochemistry (IHC) staining positive regions were less intense among the MtbPOS CoV2POS lungs compared to MtbNEG CoV2POS lungs.
The same experiments were performed with mouse-adapted CoV2 (MACoV2) infected B6 mice to assess whether CoV2 resistance was specifically seen with the ACE2 transgenic model. The study groups were – MtbNEG MACoV2POS, MtbPOSMACoV2POS, and MtbPOS MACoV2NEG.
This time, the loss of body weight in the MtbNEG MACoV2POS mice was less dramatic. No weight loss, lower viral burden, and unchanged Mtb burden in lungs were detected in the MtbPOSMA CoV2POS mice. While, the MtbNEG MACoV2POS mice exhibited increased IFIT3, IFITM3, IFNa, ACE2, and IL6. Additionally, IL6 and IFNa were high in the MtbPOS MACoV2NEG group, which remained unaffected post-challenge.
IFIT3 was expressed in both, MtbPOSMA CoV2POS and MtbNEG MACoV2POS groups, and more so in the latter group. The resistance cannot be attributed to the absence of ACE2 expression in the lungs. Moreover, the MACoV2 challenge did not affect MtbPOSMACoV2POS lungs, unlike the MtbNEG MACoV2POS group.
Furthermore, the MACoV2 resistance-associated immune environment in Mtb infected mice lungs were studied by analyzing live lung CD45+ cells in each group, 7th-day post-challenge. Out of the 12 clusters, two B-cell clusters, four T-cell clusters, one basophil cluster, one neutrophil cluster, three myeloid-cell clusters, and one natural-killer (NK) cell cluster were identified.
Although the number varied between groups, it was seen that 50% of the UI lung comprised of an innate cluster—the rest being T-cells and B-cells. T-cell cluster, B-cell cluster, MPOS cluster, and DC cluster were higher in the MtbNEG MACoV2POS lungs.
The MtbPOS MACoV2NEG lung revealed relative increases in all immune clusters at the expense of naïve T-cells, NK cells and neutrophils, compared to UI lungs. The resemblance of MtbPOSMACoV2POS, and MtbPOS MACoV2NEG lungs was comparable except CD8 memory T-cell, DC, expanded B cell, and activated B-cell clusters at the expense of naïve T-cells, NK cells, and neutrophils.
It was inferred that the resistance of MtbPOS MA CoV2POS mice depends upon the immune environment of the lungs. Except for the expanded B- and T-cell clusters, the lung immune environment of the MtbPOSMA CoV2POS mice and Mtb monoinfected lungs were similar.
Therefore, Mycobacterium tuberculosis-infected B6 mice were found to be resistant to CoV2 secondary infection. Mtb infection leads to an inflammation that makes the lungs resistant to CoV2 propagation and the resulting immunopathological responses; however, the entry of CoV2 in cells remains unaffected.
These effects may either occur due to the innate immune lineages present in the lungs after Mtb infection – which restrict CoV2; and/or the adaptive immune response elicited by Mtb that confers heterologous immunity by cross-reacting with the CoV2 antigens.
Conclusion
Hence, it was concluded that prior infection with Mycobacterium tuberculosis protects against secondary SARS-CoV-2 infection.
- Rosas Mejia, O., Gloag, E., Li, J., et al. (2022). Mice infected with Mycobacterium tuberculosis are resistant to acute disease caused by secondary infection with SARS-CoV-2. PLOS Pathogens. doi: 10.1371/journal.ppat.1010093. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1010093
Posted in: Medical Science News | Medical Research News | Disease/Infection News
Tags: ACE2, B Cell, CCL2, Cell, Coronavirus, Coronavirus Disease COVID-19, Cytokine, Genes, IHC, Immune Response, immunity, Immunohistochemistry, Inflammation, Interferon, Interleukin, Liver, Lungs, Membrane, Mortality, Necrosis, Neutrophils, Pneumonia, Propagation, Protein, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spleen, Syndrome, T-Cell, TNFα, Transgenic, Tuberculosis, Weight Loss

Written by
Nidhi Saha
I am a medical content writer and editor. My interests lie in public health awareness and medical communication. I have worked as a clinical dentist and as a consultant research writer in an Indian medical publishing house. It is my constant endeavor is to update knowledge on newer treatment modalities relating to various medical fields. I have also aided in proofreading and publication of manuscripts in accredited medical journals. I like to sketch, read and listen to music in my leisure time.
Source: Read Full Article


