Study provides preliminary evidence in favor of a new type 1 diabetes treatment
Type 1 diabetes is an autoimmune disease that causes the body’s immune system to attack and destroy insulin-producing beta cells in the pancreas. Traditional management of type 1 diabetes has primarily involved replacing the missing insulin with injections which, though effective, can be expensive and burdensome.
A new study, published in Cell Reports Medicine, led by researchers at the University of Chicago Medicine and Indiana University suggests that an existing drug could be repurposed to treat type 1 diabetes, potentially reducing dependence on insulin as the sole treatment. The study is titled “Inhibition of Polyamine Biosynthesis Preserves β-Cell Function in Type 1 Diabetes,”
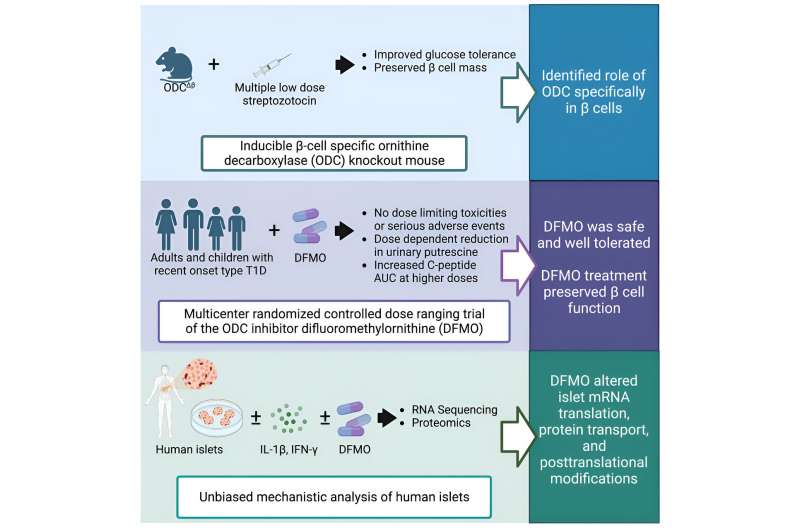
The research centers on a medication known as α-difluoromethylornithine (DFMO), which inhibits an enzyme that plays a key role in cellular metabolism. The latest translational results are a culmination of years of research. In 2010, while corresponding author Raghu Mirmira, MD, Ph.D., was at Indiana University, he and his lab performed fundamental biochemistry experiments on beta cells in culture.
They found that suppressing the metabolic pathway altered by DFMO helped protect the beta cells from environmental factors, hinting at the possibility of preserving and even restoring these vital cells in patients diagnosed with type 1 diabetes.
The researchers confirmed their observations preclinically in zebrafish and then in mice before senior author Linda DiMeglio, MD, MPH, Edwin Letzter Professor of Pediatrics at Indiana University School of Medicine and a pediatric endocrinologist at Riley Children’s Health, launched a clinical trial to evaluate the safety and tolerability of the drug in type 1 diabetes patients. The results of the trial indicated that the drug is safe for type 1 diabetes patients and can help keep insulin levels stable by protecting beta cells.
“As a physician-scientist, this is the kind of thing we’ve always strived for—to discover something at a very basic, fundamental level in cells and find a way to bring it into the clinic,” said Mirmira, who is now Professor of Medicine and an endocrinologist at UChicago Medicine. “It definitely underscores the importance of supporting basic science research.”
“It’s been truly thrilling to witness the promising results in the pilot trial after this long journey, and we’re excited to continue our meaningful collaboration,” said DiMeglio.
Importantly, DFMO has already been FDA-approved as a high dose injection since 1990 for treating African Sleeping Sickness and received breakthrough therapy designation for neuroblastoma maintenance therapy after remission in 2020. Pre-existing regulatory approval could potentially facilitate its use in type 1 diabetes, saving effort and expense and getting the treatment to patients sooner.
“For a drug that’s already approved for other indications, the approval timeline can be a matter of years instead of decades once you have solid clinical evidence for safety and efficacy,” said Mirmira. “Using a new formulation of DFMO as a pill allows patients to take it by mouth instead of needing to undergo regular injections, and it has a very favorable side effect profile. It’s exciting to say we have a drug that works differently from every other treatment we have for this disease.”
To follow up on the recently published results, first and co-corresponding author Emily K. Sims, MD, Associate Professor of Pediatrics at IU School of Medicine and a pediatric endocrinologist at Riley Children’s Health, launched a multi-center clinical trial to gather even stronger data regarding the efficacy of DFMO as a type 1 diabetes treatment.
“With our promising early findings, we hold hope that DFMO, possibly as part of a combination therapy, could offer potential benefits to preserve insulin secretion in individuals with recent-onset type 1 diabetes and ultimately also be tested in those who are at risk of developing the condition,” said Sims.
“A new era is dawning where we’re thinking of novel ways to modify the disease using different types of drugs and targets that we didn’t classically think of in type 1 diabetes treatment,” said Mirmira.
More information:
Emily K. Sims et al, Inhibition of polyamine biosynthesis preserves β cell function in type 1 diabetes, Cell Reports Medicine (2023). DOI: 10.1016/j.xcrm.2023.101261
Journal information:
Cell Reports Medicine
Source: Read Full Article