The effects of mechanical forces on musculoskeletal cells and tissues
 Thought LeadersDr. Marina DanalacheLab Leader
Thought LeadersDr. Marina DanalacheLab LeaderUniversity of Tübingen
In this interview, Dr. Marina Danalache, Lab leader at the Laboratory of Cell Biology, Department of Orthopedic Surgery, University of Tübingen, talks to News-Medical Life Sciences about the ongoing research on the effects of mechanical forces on musculoskeletal cells and tissues, as well as how these forces mediate homeostasis and drive pathophysiological processes.
Please introduce yourself and your current field of research.
Being a millennial who loves technology and is fascinated by its use in almost every aspect of life, I earned a Bachelor's and Master's degree in Biomedical Engineering. In my pursuit of discovering new things and developing new skills, I switched from industry to academia and earned a Ph.D. in Experimental Medicine from the University of Tübingen, Germany. “Innovation is the key to the future, but basic research is the key to future innovation”- Jerome Isaac Friedman, Emeritus 1990 Nobel Laureate. These words were the main driving force at the beginning of my research journey and still are, remaining deeply engraved in my mind, as I employed atomic force microscopy (AFM) as a means to examine the structure-function relationship of articular cartilage.
Currently, I am the lab leader of the Laboratory of Cell Biology, Department of Orthopedic Surgery, University of Tübingen. Our research focuses on the effects of mechanical forces on musculoskeletal cells and tissues (i.e., articular cartilage and bone), as well as how these forces mediate homeostasis and drive pathophysiological processes. We are also interested in how to modulate these forces and the impact they have on a cellular and molecular level, with the optimistic goal of one day being able to prevent, stop, and why not, even treat degenerative cartilage diseases, which are extremely common in an aging society.
 Image Credit:Shutterstock/angellodec
Image Credit:Shutterstock/angellodec
Your research is at the interface between biomedical research, biomechanics, material science, and imaging. Do you expect biomechanical properties and biomarkers, such as stiffness, to play an important role in biomedical applications?
Yes, definitely! In a clinical landscape where disease heterogeneity and interpatient variability limit diagnosis, prognosis, and treatment outcome, specific biomarkers – a portmanteau of quantifiable biological characteristics that can be measured accurately, quickly, and reproducibly – are highly sought after. Stiffness in particular is one such example of a label-free biomarker. The main advantage of evaluating mechanical properties such as stiffness is that it has a direct impact on tissue functionality. This is not only important for load- bearing tissues such as articular cartilage and bone, which can withstand enormous amounts of intensive and repetitive forces during life, it also plays a role in other pathologies. In fact, several interesting studies have shown that stiffness assessments can be used to distinguish between healthy and cancerous lesions in a variety of cancer entities in a fast, non-invasive and accurate manner.
There is undoubtedly enormous potential, not only for diagnosis and therapy, but also for the emerging field of regenerative medicine, where it is well known that the stiffness of artificial constructs is critical in regulating cell behavior and the inherent regenerative potential.
What methods do you use to investigate the biomechanical properties of biological samples and materials?
We use macro-indenters (tensile testers used to determine the tensile strength) as well as more refined methods such as fiber Bragg grating and AFM to investigate the mechanical properties of our samples. In the majority of our research projects, the magnitude and nature of the strains that cells within the tissue respond to locally were mainly investigated using AFM at both the nano- and microscale.
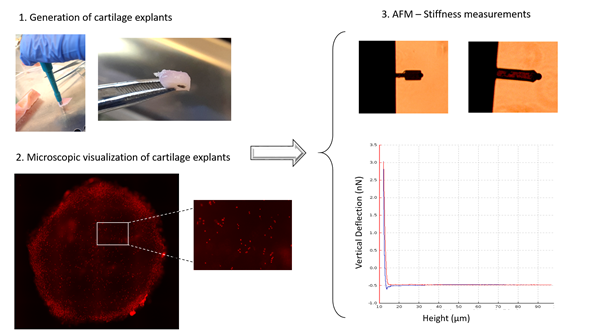
Figure 1. Flow chart of a typical experimental design for measuring cartilage stiffness.
You have published papers on the use of Atomic Force Microscopy (AFM) in the study of biomechanics. AFM is a sensitive, non-destructive, stress analysis method that enables the real-time investigation of, e.g. morphology and mechanical behavior. Where do you think AFM can make a particular contribution to your research?
As research efforts are delving deeper into the mechanisms that underlie difficult-to-treat degenerative disorders such as osteoarthritis, seeking new clues along well-trodden paths, they reopen Pandora's box of development mechanisms – single-cell approaches. To this end, AFM has definitely become a popular tool for sample/material characterization, complementing optical and electron microscopy. It can generate highly localized snapshots of mechanical properties with resolutions down to the cellular and subcellular components (nanometer and beyond). Simply put, AFM is capable of sensing the invisible. In a context where "mechano-engineered" cells/microenvironment and "mechano-active" scaffolds are the "new thing" for tissue engineering, AFM acts as a mechano-pen, allowing controlled assessment and decryption of mechanobiological patterns. In fact, due to the AFM's high sensitivity and resolution, as well as its ability to assess disease stiffness changes at an early stage, I would go so far as to say that it could potentially be used in an in vivo clinical setting if some technical issues such as device handling, shape, temperature sensitivity , etc. were resolved.
 Image Credit:Shutterstock/MarinaSantiga
Image Credit:Shutterstock/MarinaSantiga
What are the benefits of studying the impact of force, stress, and response to mechanical load, and how can these results contribute to a better understanding of clinical questions, such as the structural stress limits of orthopedic implants?
Of all organs and tissues, the musculoskeletal system is particularly exposed to constant biomechanical forces. A better understanding of the mechanobiology of these functional units also means being able to better address the associated pathologies. Beyond improving our understanding of the processes taking place, it also empowers us to design new treatment approaches that incorporate this knowledge in their design. To give a simple example, artificial biomimetic matrixes that are to be implanted in cartilage defects to aid in the repair process of new cartilage can now be produced with specific biomechanical targets in mind. Such matrixes can thus better mimic the original cartilage architecture and aid in the differentiation of mesenchymal stem cells into healthy chondrocytes.
What physical models are particularly helpful in the analysis of experimental data?
Depending on the characteristics of the indenter and the sample/material tested, various physical models are used to analyze the acquired data of biological samples. In the case of AFM, the various Hertzian Model-adapted algorithms (e.g., classical Hertz-fit, Sneddon model, Rico model, etc.) are the most established and widely used models.
Do you see the results of cutting-edge experimental research in orthopedic biomechanics being transferred to real-life bioengineering and clinical applications, e.g., in the design, development, and analysis of new biological and artificial materials? Do you expect the importance of this research to grow in the near future?
As mentioned, regenerative therapies in the musculoskeletal field will most likely benefit from our deepened understanding of the precise biomechanical properties of tissues such as cartilage, bone, and tendons. For adequate repair, these tissues are all dependent on the ingrowth of stem cells that differentiate into regular functioning specialized cells. Numerous parameters can influence this process, and an inadequate biomechanical environment will almost certainly prevent a favorable line differentiation, thus impairing the regeneration potential of these cells. Adjusting the biomechanical properties of matrixes augments, or adjunct grafts to the physiological situation improves the performance of these therapies. It's not all biomechanics, yet without a thorough understanding of biomechanics, nothing works properly.
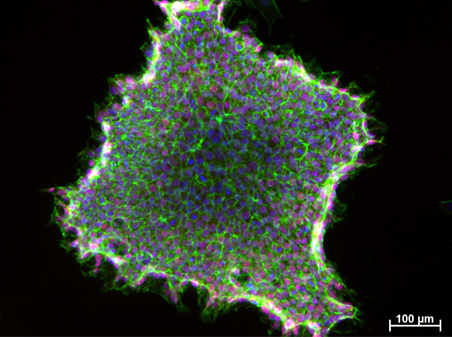
Figure 2. Induced Pluripotent Stem Cells (IPSCs) derived from jaw periosteal cells. NANOG (pluripotency marker) – red, F-actin (cytoskeleton) – green, cell nuclei – blue.
What are the main challenges you face in your work and the investigation of the structure, composition, and biomechanical properties of samples, such as cells, tissues, and biomaterials?
One of the main challenges in our research field like in any other is materializing and implementing the idea in practice. Although the development of experimental research procedures and the implementation of project designs appear to be simple and straightforward experiments, the steps involved are far more complex than one might expect. Depending on the experimental settings for mechanical testing, parameters such as selecting the appropriate indenter (i.e., AFM cantilever), preparing and fixing the sample for measurements, ensuring an optimal interaction between the sample and the indenter (especially for biological samples with uneven surfaces), and selecting the best-suited model for data analysis are all crucial aspects for AFM measurements. Once all is set, it runs smoothly, and automated measurements can be performed.
Because orthopedics is a niche field that does not receive as much attention as, say, oncology, larger studies or innovations are challenging, making those who work in the field pioneers in a narrow research area. This is also reflected in the relatively limited funding options. At the same time, working in such an environment is exciting and makes one's work more challenging in a positive way, as if you're discovering new things and making a difference in the field.
You work at the orthopedic clinic of the University Hospital of Tübingen in Germany. How does the proximity to a clinical environment benefit your research?
Working in proximity to a clinical environment has many advantages. First, obtaining the necessary tissues for our experiments is usually not a problem, and many measurements can be performed directly in human samples without the need for animal tissue. Secondly, the exchange of ideas and concepts with people treating those conditions that other researchers only read about, really broadens the horizon and can often help to develop really interesting ideas. Sometimes it's surprising to see what techniques are already in clinical use when you'd think they were still in the experimental stage, and vice versa.
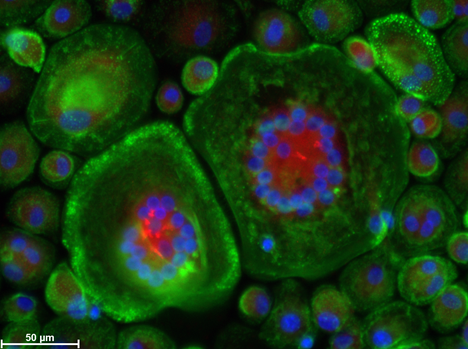
Figure 3. Human peripheral blood mononuclear cells (PBMCs) differentiated into osteoclasts. ALK (alkaline phosphatase) – red, F-actin (cytoskeleton) – green, cell nuclei – blue.
How is the field of orthopedic biomechanics changing? Is there an increased awareness of the role mechanical stress plays in normal, diseased, injured, or surgically treated bones, joints, and soft tissues?
Over the last two decades, our understanding of cartilage biology has evolved dramatically. We now recognize the regenerative potential of this tissue, as well as its limitations. Such an understanding is critical for preventive strategies, such as instructing trainers or employers on the proper conditions for long-term health in the working population. In the field of trauma surgery, we've also seen quite a revolution in terms of fractured bone healing requirements. The need for limited micromotion at a fracture site is now met through the most recent generation of osteosynthesis materials, particularly angle stable plates for internal fixation. So, as you can see, understanding biomechanics at both the cellular and tissue levels is still a very impactful and up-to-date area of study, even more so, considering the aging population and the impact of musculoskeletal diseases on society.
About Dr. Marina Danalache
Dr. Marina Danalache has a Bachelor of Science (BSc.) in Medical Engineering from the University of Medicine and Pharmacy, Lasi, Romania and a Master of Science (MSc.) in Medical Engineering from Furtwangen University, Germany. She completed her PhD in Experimental Medicine at the Orthopaedic Clinic of the University Hospital Tübingen and went on to do a Postdoc in the Orthopaedic Cell Biology Research Lab at the Orthopaedic Clinic of the University Hospital Tübingen, Germany.
Head of Laboratory of Cell Biology,
Department of Orthopedic Surgery,
University of Tübingen, Germany
[email protected]
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Source: Read Full Article


