Researchers identify ‘hot spots’ for developing lymphatic vessels
When an embryo develops, a wide variety of proteins and enzymes trigger a series of biochemical reactions. The development of the lymphatic vasculature is crucially dependent on one specific protein—the growth factor VEGF-C. In order to become biologically active and to initiate downstream signaling events, the protein must first undergo processing steps. Thus far it was unclear, however, how and where the necessary factors come together that are required for VEGF-C activation, and which cell types provide these individual factors during development.
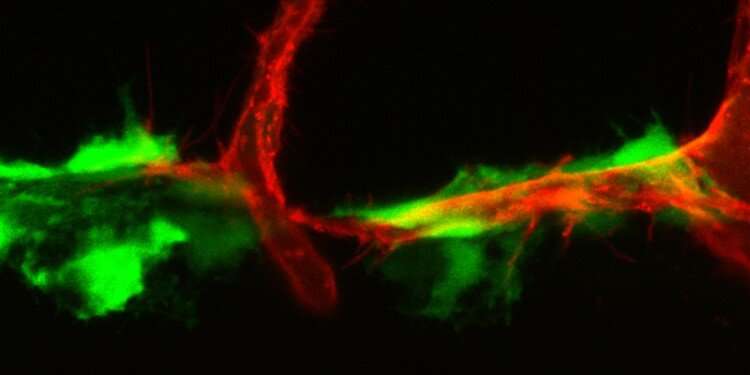
Using the zebrafish model, an international team of researchers has now gained new insights into how and at which spots the individual protagonists of the VEGF-C signaling pathway need to interact with each other in the embryo, in order to allow proper lymphatic vasculature development. The researchers identified special cells in the connective tissue that, at defined spots in the embryo, produce the important protein VEGF-C itself, as well as two protein-processing enzymes which can activate the VEGF-C protein. In the case of one of these proteins, it was previously completely unknown that it had this function.
“The cells we have identified, the fibroblasts, represent what appear to be ‘hot spots’ for the occurrence of the activated protein,” says Dr. Andreas van Impel from the University of Münster, who led the study. The results provide not only fundamental new insights into how the lymphatic system develops, but also, conversely, has relevance for human diseases. If the signaling pathway is miss-regulated in humans, genetically caused forms of lymphoedema will develop. Also, cancer cells can employ the lymphatic vessels to metastasize.” If it turns out that a comparable fibroblast population is present in humans as well, our findings could impact future approaches to treat a variety of lymphatic related diseases,” says Andreas van Impel. The study has been published in the journal “Nature Communications“.
Background and method:
Worldwide, there are around a thousand groups of researchers working with zebrafish. These fish are suitable to investigate how an organism develops—including the bones, blood vessels and the lymphatic system. As fish embryos develop outside the womb and are transparent for a few days, the researchers can precisely monitor changes in development and identify connections with humans.
In their current study, the researchers investigated the protein VEGF-C, which is important for lymphatic vessel growth. In order for it to become biologically active, it has to be processed which means parts of the protein need to be cleaved off. VEGF-C can only bind and activate its receptor once two so-called pro-domains have been removed. The enzymes which process the protein are called proteases. In their study, the researchers examined the role of the secreted proteases ADAMTS3 and ADAMTS14 during the activation of VEGF-C.
Using state-of-the-art confocal microscopy, the researchers showed that embryos lacking both proteases as a result of genetic mutations fail to form lymphatic vessels—which led the researchers to conclude that the processing of VEGF-C can be carried out not only (as previously assumed) by ADAMTS3, but also by the related protease ADAMTS14.
This was something that the researchers found not only for the zebrafish proteins, but also in experiments with human cell culture lines. “Our observations indicate that this ability of the ADAMTS14 protein is conserved through evolution,” says lead author Guangxia Wang, who is writing her Ph.D. thesis at the CiM-IMPRS Graduate School at Münster University. The School is part of the “Cell Dynamics and Imaging” research focus.
In cell transplant experiments, the researchers were able to show that certain neuronal structures, as well as fibroblasts, represent the cellular sources for both proteases and, in addition, for the VEGF-C protein. “Activating the VEGF-C protein in these positions in the embryo was sufficient to trigger normal development of lymphatic vessels, which again emphazises the importance of this cell population for the regulation of lymphangiogenesis,” says Andreas van Impel.
Source: Read Full Article


