Breathing easier with a better tracheal stent
Pediatric laryngotracheal stenosis (LTS), a narrowing of the airway in children, is a complex medical condition. While it can be something a child is born with or caused by injury, the condition can result in a life-threatening emergency if untreated.
Treatment, however, is challenging. Depending on the severity, doctors will use a combination of endoscopic techniques, surgical repair, tracheostomy, or deployment of stents to hold the airway open and enable breathing.
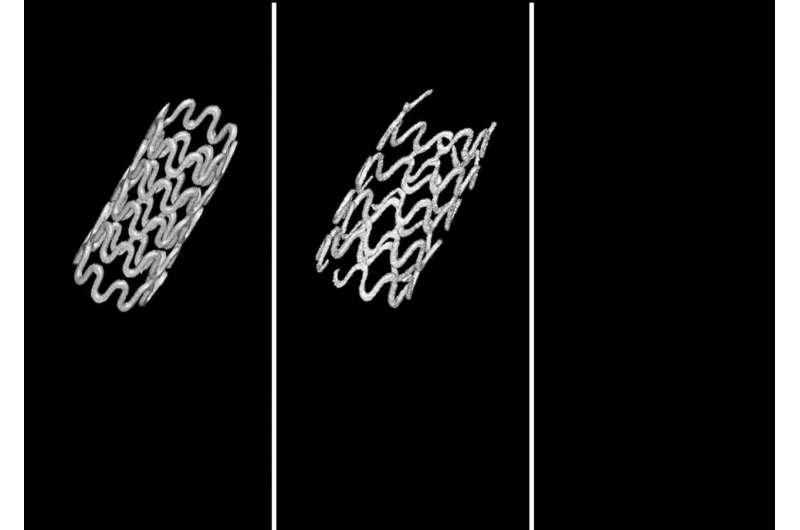
While stents are great at holding the airway open and simultaneously allowing the trachea to continue growing, they can move around, or cause damage when they’re eventually removed. New research published in Communications Biology and led by the University of Pittsburgh is poised to drastically improve the use of stents, demonstrating for the first time the successful use of a completely biodegradable magnesium-alloy tracheal stent that avoids some of these risks.
“Using commercial non-biodegradable metal or silicone based tracheal stents has a risk of severe complications and doesn’t achieve optimal clinical outcomes, even in adults,” said Prashant N. Kumta, Edward R. Weidlein Chair Professor of bioengineering at the Swanson School of Engineering. “Using advanced biomaterials could offer a less invasive, and more successful, treatment option.”
In the study, the balloon-expandable ultra-high ductility (UHD) biodegradable magnesium stent was shown to perform better than current metallic non-biodegradable stents in use in both in lab testing and in rabbit models. The stent was shown to keep the airway open over time and have low degradation rates, displaying normal healing and no adverse problems.
Source: Read Full Article


