Scientists discover a combination therapy for aggressive brain tumors
Glioblastomas are the most common and most aggressive brain tumors. Their survival rate has barely increased over the last 50 years, indicating an urgent need to develop new therapeutic strategies. In a paper published this week in the journal Molecular Cancer Therapeutics, a journal of the American Association for Cancer Research, the team led by Massimo Squatrito, head of the Seve Ballesteros Foundation Brain Tumor Group at the Spanish National Cancer Research Center (CNIO), proposes a novel therapeutic strategy based on the combination of temozolomide, the first-line treatment for these patients, and dianhydrogalactitol, a drug that is being tested in clinical trials and is already approved for the treatment of other tumors.
Currently, the main and virtually only treatment for glioblastomas is the combination of radiotherapy and the chemotherapy agent temozolomide, after surgical resection of the tumor mass. Like most chemotherapeutic agents used, temozolomide induces damage to the genetic material of tumor cells, causing them to break down and die. However, in almost half of the patients, such tumors become resistant to the drug and the tumor continues to grow even while undergoing treatment.
“Although the incidence of glioblastoma is not excessively high in adults, they are the most common brain tumors, and there are no effective treatments or markers of response to treatment or of the generation of resistance,” says Squatrito.
DNA repair, the basis of resistance
Why do patients with glioblastoma stop responding to temozolomide? Squatrito and his team shed light on this central question last year in a paper published in the journal Nature Communications: some glioblastomas produce genomic rearrangements in the DNA repair gene MGMT, which increases its production and repairs the DNA damage induced by temozolomide so that the tumor manages to grow and evade treatment.
In the paper published now, the researchers studied temozolomide resistance in depth by using glioblastoma cell lines in which several key genes were silenced. The results show that this resistance not only depends on the MGMT gene but that it may also be mediated by failures in the MMR (DNA repair) pathway so that when any of its components are altered, tumor cells accumulate mutations that give them the ability to evade the effects of temozolomide.
Tumors are complex systems that use multiple tools to trick the body into supporting their growth and development. Combination therapies, targeting several components involved in the tumor process, are a revolution that brings hope to many patients. Advances in the understanding of the molecular biology of tumors allow the emergence of new therapies and targeted combinations thereof to fight the tumors and to avoid any type of resistance they may develop.
Combination therapy with dianhydrogalactitol
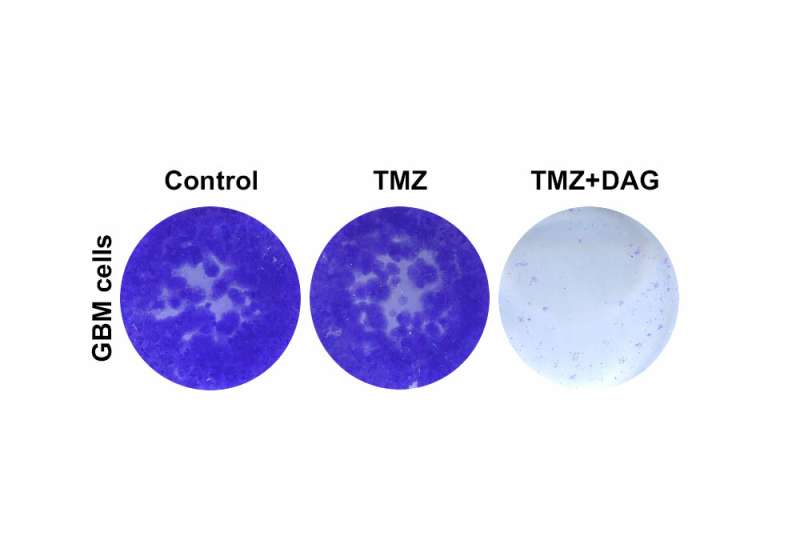
In the present study, the researchers focused on the drug dianhydrogalactitol, a chemotherapeutic agent that is able to cross the blood-brain barrier and reach the central nervous system, where it induces DNA damage in tumor cells. Dianhydrogalactitol is currently being tested in clinical trials for gliomas and other types of cancer such as ovarian cancer and is already approved in China for the treatment of acute myeloid leukemia and lung cancer.
The results of this study show that temozolomide and dianhydrogalactitol act synergistically on tumor cells in vitro, resulting in slower growth of these cells compared to when they are treated with each drug individually. The researchers observed similar results in mice with brain tumors, which survived longer when treated simultaneously with temozolomide and dianhydrogalactitol.
Furthermore, the results suggest that, unlike temozolomide, the anticancer effects of dianhydrogalactitol are independent of the MGMT DNA repair gene and the MMR pathway. “Our data show that dianhydrogalactitol could be an effective treatment that circumvents the resistance mechanisms that arise during temozolomide treatment,” explains Miguel Jiménez-Alcázar, first author of the paper.
Source: Read Full Article


