‘Social cells’ related to social behavior identified in the brain
A research team led by Professor Takumi Toru of Kobe University’s Graduate School of Medicine (also a Senior Visiting Scientist at RIKEN Center for Biosystems Dynamics Research) have identified ‘social cells’ in the brain that are related to social behavior. The cells were identified via Ca imaging conducted using a microendoscope. It is expected that further research will illuminate the neural network for decision-making in social behavior.
These research findings were published in the American scientific journal PLOS Biology on September 21.
Social distancing is one of the methods that is being endorsed to prevent widespread novel coronavirus infection. It has been said that a physical distance of 2 meters is necessary to prevent virus transmission. How are distances between people, socially distanced exchanges and societal interactions determined?
This kind of social behavior is a complex behavioral pattern consisting of ‘sensory inputs’, ‘internal states’ and ‘decision making’ that span the stages from ‘sensory processing’ to ‘behavior modification’. The behavioral pattern sequence begins with individuals using multimodal sensory cues (such as sight, smell, hearing and touch) to process information about another individual. This information is then referenced against (social) internal states (such as motive, arousal, emotion, interoception and memory) and behavior is decided upon (for example, investigative, mating, aggressive, nurturing or dominating behaviors).
It is understood that regions of the brain such as the amygdala, pituitary, mesencephalon and the prefrontal cortex are involved in social behavior. However, the detailed neural networks of these regions are not yet well understood, particularly at a cellular level.
Methods to map neural activity across the entire brain have been developed. These include functional MRI and mapping the expression of immediate-early genes, such as c-fos. In addition, specific neural network regions in freely moving mice have been monitored by methods including fiber photometry and Ca imaging conducted using a miniaturized fluorescence microscope affixed to the rodent’s head (as in this study), in addition to observations conducted with a two-photon microscope on mice with their heads fixed in place.
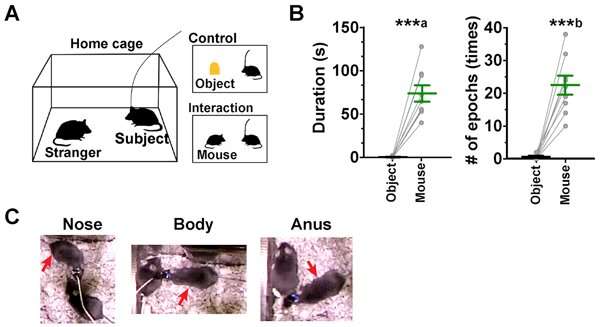
A test mouse was placed in a ‘home cage’ and its behavior towards either a stranger mouse (that it had never come into contact with before) or a static object placed in the same cage was observed. Both the duration and frequency of interactions between the test mouse and stranger mouse were significantly greater than the test mouse’s interactions with the static object. The test mouse demonstrated social behaviors, including contact with the nose, body and anus of the stranger mouse.
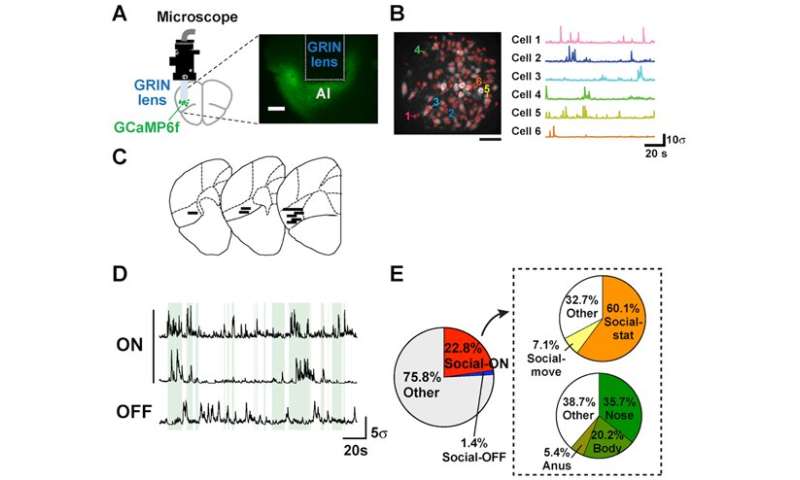
The research team injected Ca indicator (GCaMP) into the insular cortex of mice using an adeno-associated virus vector. Next a GRIN lens was inserted into the agranular insular cortex (AI). This enabled the research team to use Ca imaging conducted via the miniaturized fluorescence microscope fixed to each mouse’s head to record the neural activity of freely moving mice.
Cellular recordings of the nerve cells (neurons) of nine mice were analyzed. Through this analysis, two types of neurons were identified; Social-ON cells, the activation of which corresponds with social interactions, and Social-OFF cells, which are active when there is no social interaction. Out of a total of 737 neurons, Social-ON cells accounted for 22.8% (168 cells) and Social OFF cells made up 1.4% (10 cells). When the test mouse was stationary during social interaction, 60.1% of the 168 Social-ON cells were active. On the other hand, 7.1% were active when the mouse was moving during social interaction. In addition, the percentage of active Social-ON cells in the test mouse correlated with different types of contact with the stranger mouse (nose-to-nose contact: 35.7% of Social-ON cells active, contact with body: 20.2%, and contact with anus: 5.4%) (see Figure 2E).
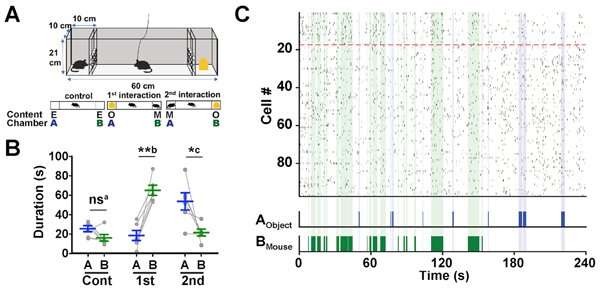
Next a different test was conducted where a mouse was placed in a rectangular chamber, with two smaller divided off sections (Section A and B respectively) at either end (see Figure 3A). For the control experiment, Sections A and B were left empty. For the 1st Interaction experiment, a static object was placed in Section A and a stranger mouse was placed in Section B. For the 2nd Interaction, the positions of the object and stranger mouse were reversed. In each experiment the test mouse’s behavior was observed for 4 minutes and the difference in the duration of social interaction (contact) was investigated for each experiment. In the control experiment, there was no difference in the time spent between Sections A and B. However, in the both 1st and 2nd Interaction experiments, the test mouse had a longer contact time with the section that contained the stranger mouse (1st Interaction: Section B, 2nd Interaction: Section A).
Moreover, the researchers were able to identify Social-ON cells (that responded to the presence of the stranger mouse) and Social-OFF cells (active during periods of no social interaction) through analysis of the neurons inside the AI. These cells reacted to the stranger mouse, regardless of whether it was placed in Sections A or B.
The insular cortex is anatomically situated to integrate multimodal sensory social cues into the ‘Social decision-making network’, which consists of the ‘social behavior network’ and the mesolimbic reward system. This research succeeded in directly observing AI activity during social interaction at a single cell level, providing new insight into the cellular basis of the insular cortex’s social functions. In other words, the researchers identified a large number of Social-ON cells and a smaller number of Social-OFF cells that demonstrated opposing activity during social interactions. Further research to identify and manipulate the activity of Social-ON and Social-OFF cells’ projection targets is expected to advance our understanding of them at a circuitry level.
Furthermore, this discovery regarding the characteristics of AI neurons implies that the insular cortex can induce a bottom-up process in response to salient stimuli (strong sensory stimuli). This result corresponds with knowledge accumulated so far regarding how the insular cortex acts as an interface between social and emotional modules in the ‘social decision-making network’.
Source: Read Full Article


